
ASH25 Day 3: Don’t Miss The Highlights
The American Society of Hematology Congress 2025 (ASH25) is officially underway in Orlando, Florida, from December 6 to 9.
It aims to bring together hematology professionals from across the globe.
Throughout the meeting, experts will present new research, clinical breakthroughs, and innovations that are defining the future of hematologic care.
Find the highlights from Day 3
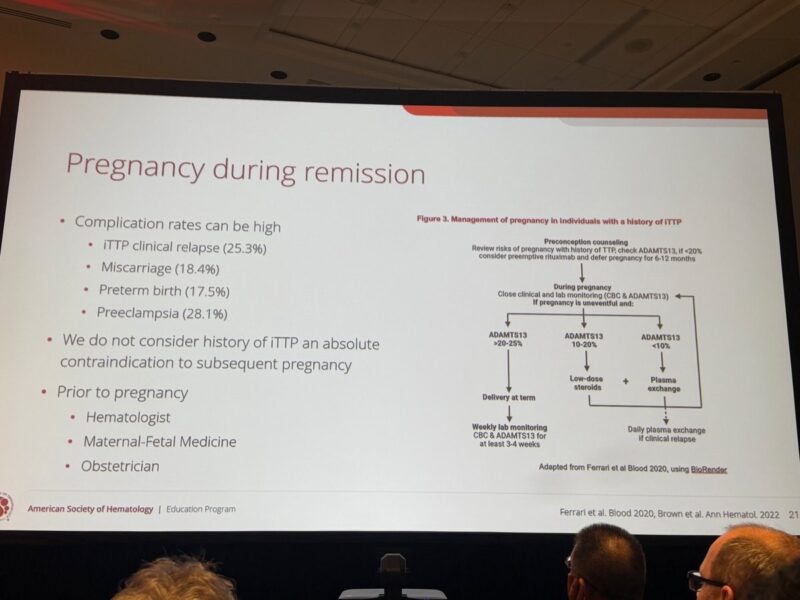
”iTTP and Pregnancy: Moving from “Contraindication” to “Careful Planning”
Is a history of immune-mediated TTP (iTTP) an absolute contraindication for pregnancy?
The answer from ASH25 is a resounding no—but it requires precision medicine and a team approach.
In the second part of his excellent education session, Dr. Senthil Sukumar (Baylor College of Medicine) tackled the complex landscape of pregnancy during remission.
Here are the critical clinical pearls for managing our patients who wish to conceive:
The Risks are Real but Manageable
We cannot sugarcoat the data. Complication rates remain high:
- Preeclampsia: ~28%
- Clinical Relapse: ~25%
- Miscarriage: ~18%
The “Pre-Conception” Window is Vital
Success starts months before conception.
Dr. Sukumar’s expert opinion suggests:
Check ADAMTS13 6–12 months prior to planned conception.
Target: If ADAMTS13 is <20%, consider preemptive rituximab and defer pregnancy for 6–12 months to ensure a safe baseline.
During Pregnancy: A Tiered Strategy
Management shouldn’t be static. The presented algorithm (adapted from Ferrari et al. and Brown et al.) proposes adapting therapy based on ADAMTS13 levels:
- >20-25%: Close monitoring (CBC and ADAMTS13 every 3-4 weeks).
- 10-20%: Consider low-dose steroids.
- <10%: Consider plasma exchange to prevent overt relapse.
Prophylaxis: Low-dose aspirin after 12 weeks is recommended (per USPSTF guidelines for high-risk preeclampsia).
The takeaway?
With a multidisciplinary team (Hematology + Maternal-Fetal Medicine) and enhanced monitoring, we can support our iTTP patients in building their families safely.
 ”
”
”Congratulations to Dr. Mysa Saad on her first oral presentation at ASH25!
Does concurrent antiplatelet or NSAID use increase bleeding risk in cancer patients receiving apixaban thromboprophylaxis?
Key Findings:
• Among patients receiving prophylactic apixaban, concurrent antiplatelet/NSAID use significantly increased clinically relevant bleeding (HR 1.78) and CRNMB (HR 1.98).
• No reduction in VTE was observed (HR 0.60).
• In subgroup analyses:
– Antiplatelet agents were the main driver of increased bleeding (CRNMB HR 2.59).
– NSAIDs did not show a significant impact on bleeding or VTE.
• Mortality outcomes were unaffected.
Implications:
These results highlight the need to carefully reassess antiplatelet/NSAID indications and conduct individualized bleeding risk evaluations when considering apixaban prophylaxis in cancer outpatients.
 ”
”
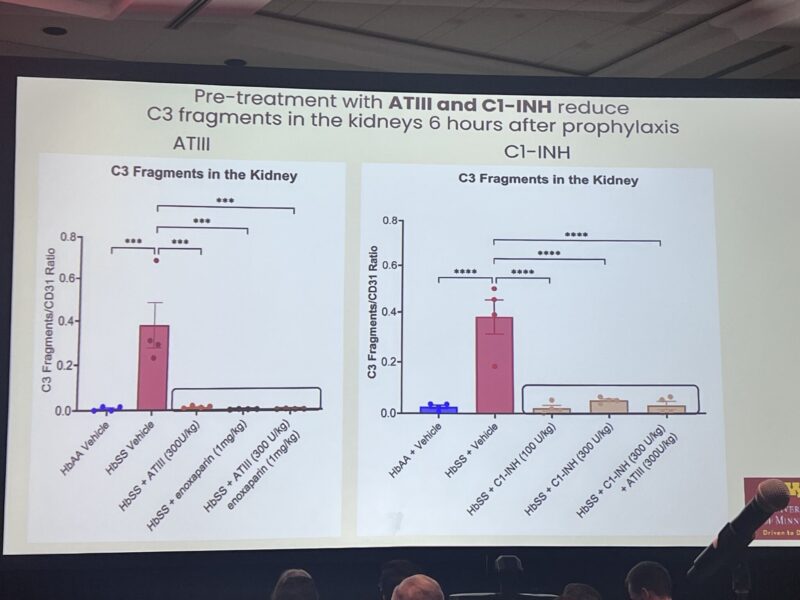
”John Belcher describes use of AT3 and C1 esterase inhibitor (C1-INH) concentrates on induced vaso-occlusion in sickle cell mice-dose réponse effect of AT3 and C1-INH and deposition of C3 in glomeruli
 ”
”
”A day filled with remarkable science in clinical development for Sickle Cell Disease at the American Society of Hematology 2025 national meeting!
Using novel new ways to positively affect the Erythron, three new approaches were discussed :
1. Transcriptome guided novel target by affecting NED Dlyation through DCN1 pathway thereby augmenting fetal hemoglobin rise ,predicted to raise fetal hemoglobin close to gene therapy ranges by just an oral drug delivery platform.
2. Knockdown of G-CSF in Sickle cell Disease , hoping it will impact Netosis and vaso occlusion.
Thromboinflammation front and center.
Very complimentary to our work being done by the Sickle Cell Hemostasis Taskforce
3. Acute chest syndrome acute treatment by reduction of NETs
Additionally proud to have shared our clincial work on standadizing point of care testing with near 100% concordance with HPLC for all hemoglobinoapthies in the USA for the first time.
 ”
”
”Just published online and presented at ASH25: EPCORE CLL-1
Epcoritamab monotherapy for Richter transformation (EPCORE CLL-1): findings from a single-arm, multicentre, open-label, phase 1b/2 trial
Great presentation by Arnon P. Kater!
Read the full article here.
 ”
”
”Huge congratulations to Dr. Abdulrahman Al Raizah on delivering his first ASH presentation!
His team’s quality-improvement project tackled two major challenges in IVC filter use: insertion without proper indication and delayed retrieval.
Through a coordinated, system-level strategy, they achieved:
• Appropriate filter use: 72.7% → 91.4%
• Retrieval among eligible patients: 76% → 92.3%
• Median retrieval time: reduced from 35 to 22 days
A fantastic example of how evidence-based policy, education, and workflow redesign can meaningfully improve thrombosis care.
 ”
”
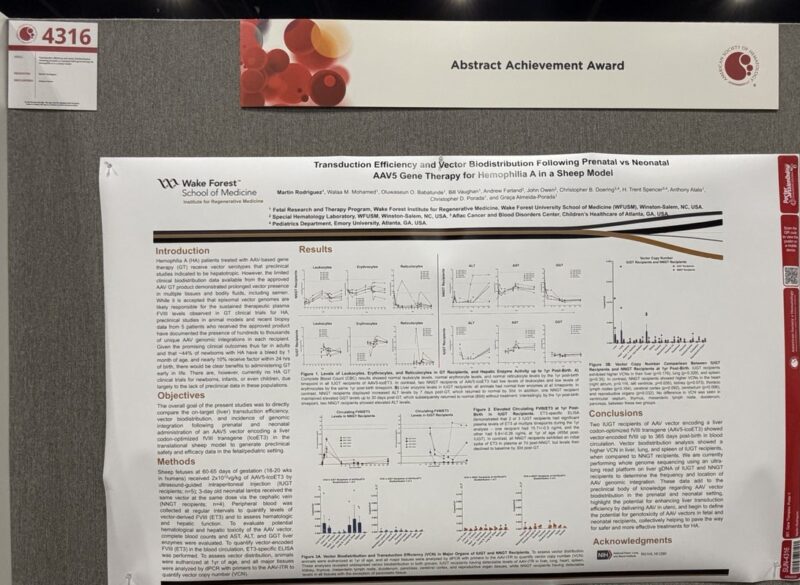
”Prenatal gene therapy for Hemophilia A shows promise!
In a sheep study, delivering AAV-based therapy before birth led to sustained FVIII levels for a year—far better than postnatal treatment.
 ”
”
”More than 800,000 people worldwide live with hemophilia.
For those who develop inhibitors, standard treatments no longer work.
At ASH25, we presented Phase 3 data, published in Blood Journals Portfolio, that could change that.
Grateful to our teams behind this remarkable science.”
”New insight
MGUS can do more than signal plasma cell disorders—it may trigger Acquired Hemophilia A, a rare autoimmune attack on Factor VIII causing severe bleeding.
Heightened vigilance is key.
 ”
”
”This year, the Sanofi rare disease team is focused on driving meaningful progress in rare blood disorders through:
Deep scientific exchange advancing knowledge in hemophilia, ITP, and iTTP
Multiple poster presentations showcasing data from our research into rare blood disorders
Clinical trial recruitment initiatives across our rare blood disorder portfolio
A highlight was the STAR (Sanofi Trial Awareness and Recruitment) program on December 6th, designed to streamline clinical study engagement, strengthen healthcare professional partnerships, and enhance diverse patient enrolment through meaningful scientific collaboration.
Grateful to our distinguished expert speakers—Dr. Guy Young, Dr. Waleed Ghanima, and Dr. Biree Andemariam—for sharing valuable insights on Sanofi’s key clinical trials in Hemophilia, wAIHA, ITP and Sickle Cell Disease.
A special thank you to the exceptional Global Sanofi Medical Affairs team, including Shariq Ali, James Cobb, Jennifer Dumont, Monique Bidell, Lauriaselle Afanador, Sandra Casiano, Chase Lee, Marta Borchiellini, K. Amy Chen, Julie Kissell Pablo Manuel Bianculli Maria Belen Rodriguez Mario Aguiar Rahul Bohra Sequoia Baskind for making this impactful event possible.
Our US Medical Colleagues Brad Ward; Dina Issa and many others.
Our Medical Colleagues from around the globe Jasmine Chang, Kevin Chueh, Marlene Gharib, Özgur Pektas, BoSkjoedt Rafn, Aymeric Duvivier, Shinichi Nakamuta, Mark Surka, Yuka Tanaka, Haruhi Ando
 ”
”
More from ASH25 featured in Hemostasis Today.
-
Jan 24, 2026, 15:54Nicolas Nesseler on How Common and Serious is Post-Op Bleeding After Heart Transplantation
-
Jan 24, 2026, 15:43Roey Tagansky on Tu Youyou and the Rediscovery That Defeated Malaria
-
Jan 24, 2026, 15:27Mavis Agnes Kisakye on Community Impact at HFU
-
Jan 24, 2026, 15:15Jo Jewell Announces Novartis Foundation and Novo Nordisk Collaboration
-
Jan 24, 2026, 15:02Todd Davenport on The Impacts of Long COVID
-
Jan 24, 2026, 14:47Seth D Ginsberg: Last Month, Something Meaningful Happened in Tokyo
-
Jan 24, 2026, 14:33Heghine Khachatryan on New Insights Into Bleeding Mechanisms in Acquired Hemophilia A
-
Jan 24, 2026, 14:21Mamta Soni Shares Highlights from CBC Chennai 2026
-
Jan 24, 2026, 13:52Larysa Mykhailova on Characterization of Allogeneic Platelet Gel
