
Transforming FVIII Product Classification: AUC and TTR as Game-Changing Metrics for Next-Generation Hemophilia A Therapy
Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital, has recently shared a very informative post on X:
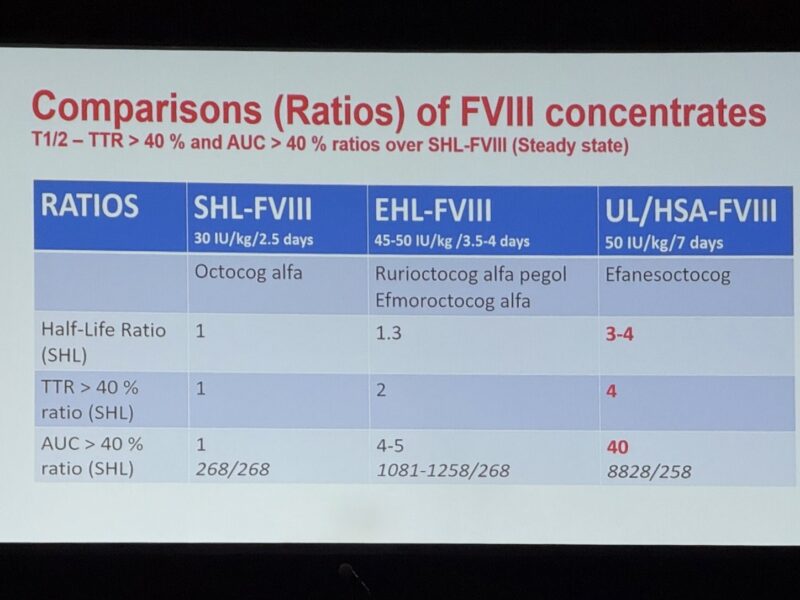
‘‘Novel FVIII Classification: Scientific Framework for Next-Generation Haemophilia Treatment. Two pharmacokinetic metrics that can redefine how we classify Factor VIII products, presented by Professor Cedric Hermans at ISTH SSC FVIII/FIX session.
The Scientific Challenge
Traditional “standard” vs “extended” half-life classifications have proven inadequate for new generation FVIII products. Recent advances have led to improved ability to maintain FVIII levels within normal range.
New Classification Metrics
– Area Under the Curve (AUC) > 40%
Measures total drug exposure over time, providing comprehensive assessment of sustained FVIII activity rather than peak-focused measurements
– Time in Target Range (TTR) > 40%
Evaluates percentage of time patients maintain therapeutically effective FVIII levels, offering crucial insights into sustained haemostatic protection
Comparative Performance Data
– Standard Half-Life (SHL-FVIII): Baseline comparators (AUC ratio: 1, TTR ratio: 1)
– Extended Half-Life (EHL-FVIII): 4-5x AUC improvement, 2x TTR enhancement
– Ultra-Long/High Sustained Activity (UL/HSA-FVIII): 40x AUC improvement, 4x TTR boost
Products like efanesoctocog alfa demonstrate remarkable sustained activity, maintaining mean FVIII levels >40 IU/dL for 4 days with geometric mean half-life of 43.3 hours
Future Integration
Further validation can be warranted for integration into routine clinical evaluation and PK tools like WAPPS-Hemo, MyPKFit, and Florio. These platforms enable Bayesian forecasting of individual PK profiles for personalized treatment optimization.
As the field evolves toward normal hemostasis as the treatment goal, these metrics provide an important scientific basis for evaluating innovative therapies.”
Stay updated with Hemostasis Today.
-
Feb 25, 2026, 16:47Alan Nurden: Platelet Defects Explain Bleeding in EHDS Patients
-
Feb 25, 2026, 16:40Mohammed Almohammadi: Uniting Leaders in Laboratory Hematology at the 1st Saudi ISLH Joint Conference
-
Feb 25, 2026, 16:37Salihu Asimawu: Inflammation Is Not a Disease – Hats Off to the Heroes Inside Us
-
Feb 25, 2026, 16:34Michael Makris: Biomarin Has Decided to Withdraw Roctavian from the Market
-
Feb 25, 2026, 16:25Tagreed Alkaltham: The Blood Banker Personality
-
Feb 25, 2026, 16:17Sanjay Ahuja: Insightful Talk on 100 Years of VWD by Jorge Di Paola
-
Feb 25, 2026, 15:20Michael Shapiro։ Creating Real Pathways for Trainees in Preventive Cardiology
-
Feb 25, 2026, 15:15Ajay Kumar: Plasma Component Quality Control Standards
-
Feb 25, 2026, 15:09Wolfgang Miesbach: Efficacy, Safety and Thrombosis Signals in Haemophilia A/B with Inhibitors

