
OCEANIC-STROKE Revives Optimism for Factor XI Inhibitors
Davide Capodanno, Professor of Cardiology at University of Catania, editor-in-Chief of EuroIntervention, shared on X:
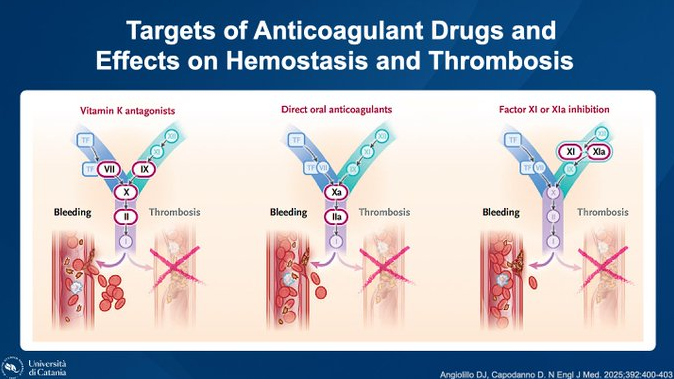
”Finally, some good news for Factor XI inhibitors, a class of anticoagulants that was starting to look genuinely unfortunate—full of promising hypotheses and remarkably short on outcomes. OCEANIC-STROKE is positive, according to a press release issued today, and notably soon after the announcement that LIBREXIA-ACS had been stopped for futility. Just as the last nail seemed ready to seal the coffin, the lid reopened and out came asundexian—the same agent for which, after OCEANIC-AF, we had essentially already recited the de profundis.
According to the press release, OCEANIC-STROKE met its primary efficacy and safety endpoints: asundexian 50 mg once daily significantly reduced the risk of ischemic stroke compared with placebo, on top of antiplatelet therapy, in patients after a non-cardioembolic ischemic stroke or high-risk TIA. There was no increase in ISTH major bleeding with asundexian.
So the same drug that failed against apixaban in cardioembolic stroke succeeds against placebo in non-cardioembolic stroke (on a background of antiplatelet therapy), highlighting the fundamentally different nature of thrombi in these two conditions.
It is also notable—judging from this sequence of press releases—that factor XI inhibition currently appears more effective in ischemic stroke (asundexian) than in acute coronary syndromes (milvexian), a scenario almost inverted compared with expectations only a few weeks ago. And several shots remain in the chamber (LIBREXIA-STROKE with milvexian; LILAC, ASTER and MAGNOLIA with the monoclonal antibody abelacimab).
OCEANIC-STROKE press release here.
LIBREXIA-ACS press release here.
Find more posts on Hemostasis Today.
-
Feb 22, 2026, 14:16Ilenia Calcaterra: From Representation to Intellectual Independence in Women in Science
-
Feb 22, 2026, 13:27Pete Stibbs: New AHA and ACC PE Guidelines Finally Align with Real Clinical Practice
-
Feb 22, 2026, 10:39Tagreed Alkaltham: Fibrinogen Concentrate Is a Deliberate Clinical Choice in Acute Bleeding
-
Feb 22, 2026, 09:38Abdulrahman Nasiri: Significant Shifts In The 2026 AHA/ACC Guidelines for Acute Pulmonary Embolism
-
Feb 22, 2026, 09:22Shiny K. Kajal: Not All Transfusion Reactions Are Immunohematologic Incompatibilities
-
Feb 22, 2026, 09:12Arun V J։ The Hidden Risks in Every Blood Bag
-
Feb 22, 2026, 08:56Parandzem Khachatryan։ How Hard Is It to Be a Mom, a Wife, a Professor, and a Doctor All at Once?
-
Feb 22, 2026, 08:46Anirban Sen Gupta Presents Bioinspired Platelet Surrogates at MTEC
-
Feb 22, 2026, 08:31Heghine Khachatryan: Advancing Care for Women and Girls with Bleeding Disorders is A Matter of Equity

