
Abdul Mannan: Rethinking Myeloid Precursor Disease in 2025
Abdul Mannan, Consultant Haematologist at Betsi Cadwaladr University Health Board, posted on LinkedIn:
”From Observation to Interception: Rethinking Myeloid Precursor Disease in 2025
Over the past few years, clonal haematopoiesis has moved from being a “benign curiosity of ageing” to a genuinely important clinical entity. The more we study it, the clearer it becomes that these precursor states don’t just sit quietly in the background. They shape two major fronts of disease: progression to MDS/AML and cardiovascular inflammation.
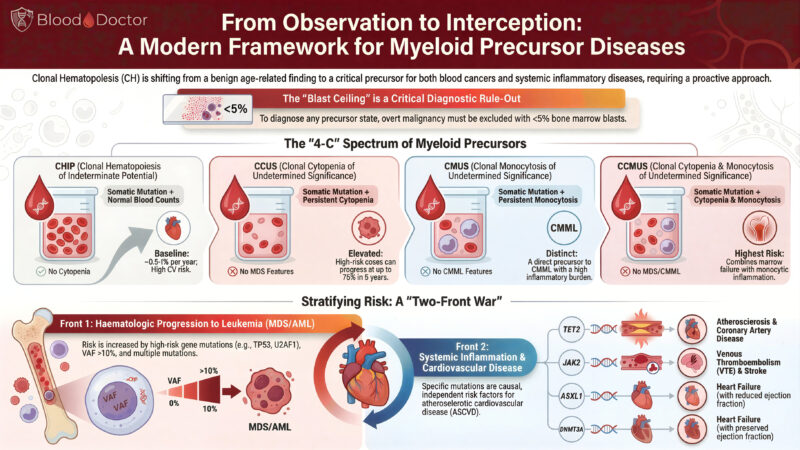
I’ve put together this infographic to make sense of the evolving landscape and the “4-C” spectrum we now recognise: CHIP, CCUS, CMUS, and CCMUS.
Why this matters
We’re no longer just observing clones; we’re learning when they behave, when they misbehave, and when they need closer follow-up. The old focus on blasts and cytopenias alone doesn’t cut it anymore. There’s a whole inflammatory story unfolding underneath.
Key points from the graphic
CHIP
Somatic mutation, normal counts, no cytopenia. Often found incidentally. Low annual progression risk, but a surprisingly strong link to cardiovascular disease. The heart always wants a cameo role.
CCUS
Somatic mutation plus persistent cytopenia. No MDS-defining features, but some cases progress rapidly. Think of this as CHIP that has decided to cause trouble.
CMUS
Somatic mutation plus persistent monocytosis. This sits on the path towards CMML and carries a high inflammatory burden. A reminder that monocytes are never shy when inflammation is involved.
CCMUS
Cytopenia + monocytosis + mutation. Highest overall risk. The “all-in-one” precursor deserves careful attention.
A Two-Front Battle
The infographic highlights what we’re all seeing in clinics and literature:
1. Front 1: Haematological progression
High-risk mutations (TP53, U2AF1, splicing genes) and VAF >10% increase the likelihood of evolving into MDS/AML.
2. Front 2: Systemic inflammation and cardiovascular disease
Mutations in TET2, JAK2, ASXL1, DNMT3A are themselves independent risk factors for:
• coronary artery disease
• venous thrombosis and stroke
• heart failure (both reduced and preserved EF)
It’s not often that haematology, cardiology, and immunology all fight for space on the same slide.
Where this takes us
We’re shifting from passive observation to interception. Early identification, structured follow-up, mutation-informed risk stratification, and collaboration across specialties will define the next decade of care.
If you’re teaching trainees, designing services, or thinking about where haematology is heading, this framework gives a clean, practical way to have those conversations.
Happy to share the high-resolution version, teaching notes, or a breakdown for your MDT or journal club.
Keep moving – one clone at a time.”
Stay updated with Hemostasis Today.
-
Jan 25, 2026, 15:57Céline Chapelle Shares Clinical Predictors From the API-CAT Trial
-
Jan 25, 2026, 15:42Francesco Lo Monaco on Heart Disease Starting Quiet While Your Labs Speak First
-
Jan 25, 2026, 15:33Wilfried Dinh: Why Heart Patients Still Have Events Despite ”Optimal” Cardiovascular Therapy
-
Jan 25, 2026, 15:25Muhammad Ibrahim on Efficacy and Safety of Extended DOACs Use in VTE
-
Jan 25, 2026, 15:08Tushar Pandey on Managing Thrombotic Thrombocytopenic Purpura
-
Jan 25, 2026, 14:55Carolina Contreras Cuevas Shares a Nationwide Study on VTE in PAD
-
Jan 25, 2026, 14:40Jeannie Devereaux Links PRP and Physical Therapy
-
Jan 25, 2026, 14:25Heghine Khachatryan on Anticoagulation After AFib Ablation: New Evidence from NEJM
-
Jan 25, 2026, 12:42Lale Tokgözoğlu on Cardiovascular Risk Factors in Women
