
12 Posts Not to Miss From the ASH 2025 – Wolfgang Miesbach
The American Society of Hematology Congress 2025 (ASH25) is officially underway in Orlando, Florida, from December 6 to 9.
It aims to bring together hematology professionals from across the globe.
Throughout the meeting, experts presented new research, clinical breakthroughs, and innovations that are defining the future of hematologic care.
Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital, shared following posts from ASH 2025 on LinkedIn:
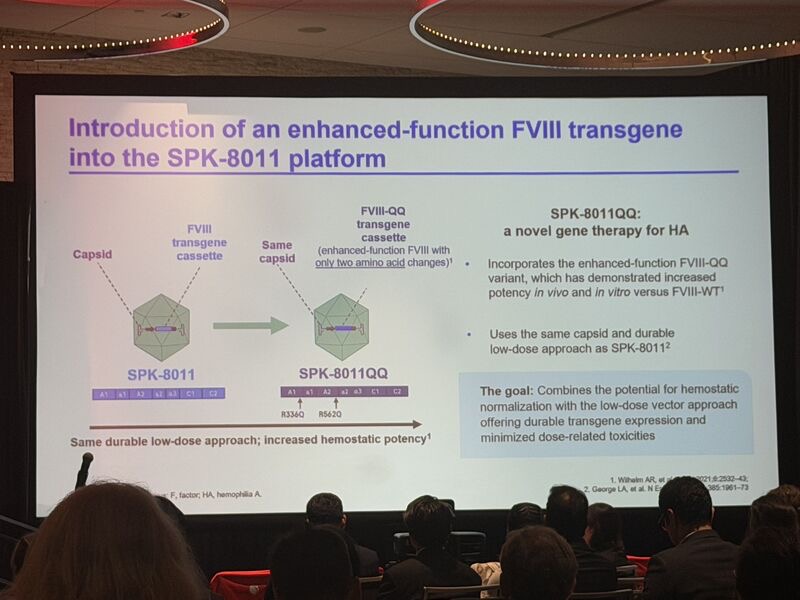
1.”Less is more: How enhanced‑function FVIII‑QQ can optimize a low‑dose gene therapy approach for haemophilia A.
Great ASH presentation by Nina Frey, PhD. SPK‑8011QQ leverages the established SPK‑8011 capsid (dirloctocogene samoparvovec), which in Phase 1/2 trials demonstrated:
- Up to 6.5 years of durable FVIII expression within the mild HA range
- No wide expression variability across time points
- No sustained or late ALT elevations
- Shortened corticosteroid exposure with IVMP; return to prophylaxis in only 5 of 25 participants
- Now combining this proven durability and safety profile with the enhanced functional potency of FVIII‑QQ.
Introducing SPK‑8011QQ: This novel construct incorporates FVIII‑QQ – an enhanced‑function FVIII variant with just two amino‑acid substitutions (R336Q and R562Q) that:
- Confer resistance to activated protein C (aPC) cleavage
- Preserve physiological A2‑domain dissociation (maintaining natural control mechanisms).
Enhanced potency in the presence of aPC:
– FVIII‑QQ retained ~65% residual activity in chromogenic assays (vs. ~43% for FVIII‑WT) at 10 nM aPC
– Significantly higher residual peak thrombin across aPC concentrations in thrombin generation assays (p=0.0018 at 4 nM)
– 80–100% FVIII activity equivalent in mouse ex vivo plasma, with markedly enhanced thrombin generation vs. FVIII‑WT
Superior haemostatic control at lower FVIII activity levels:Tail‑clip bleed model: FVIII‑QQ achieved enhanced bleed control at markedly lower FVIII levels than FVIII‑WT, with significantly reduced blood loss (p=0.0089 and p=0.0079)
Encouraging preliminary safety profile:
Ferric chloride thrombosis model: FVIII‑QQ showed a lower incidence of animals reaching the near‑occlusive zone (>85% blood flow reduction) vs. positive control
A Phase 2b study by Roche is now underway to translate these preclinical insights into meaningful clinical benefit.
 ”
”
2.”From Daily Pills to Remission after Short‑Course Therapy in ITP
New data from the phase 3 VAYHIT2 trial in primary immune thrombocytopenia (ITP), presented yesterday at ASH 2025 by Hanny Al-Samkari and published simultaneously in NEJM.
In VAYHIT2, adults with primary ITP and insufficient response or relapse after first‑line steroids were randomized to ianalumab (3 or 9 mg/kg) or placebo, all on background eltrombopag.
A short course of four once‑monthly intravenous infusions of ianalumab led to markedly better disease control and allowed many patients to come off daily therapy while maintaining safe platelet counts.
Key efficacy observations:
· Around half of patients on 9 mg/kg remained in remission at 12 months·
· Higher rates of stable platelet responses during and after eltrombopag taper
Why the mechanism is interesting:
Ianalumab is an anti‑BAFF receptor monoclonal antibody, directly targeting B cells and the autoimmune process that underlies ITP.
By combining B‑cell modulation with a thrombopoietin receptor agonist and then withdrawing both, the strategy aims not only to raise platelets but to reset the immune system and achieve more durable responses off continuous therapy.
What this could mean for practice:
For many years, second‑line ITP treatment has largely meant indefinite daily medication or serial switches between therapies.
A finite, four‑infusion regimen that can induce remission with treatment discontinuation would represent a meaningful shift in how clinicians and patients think about long‑term management and goals of care.
Safety considerations:
The safety profile in VAYHIT2 was broadly consistent with expectations:
· No apparent excess in serious infections despite profound B‑cell depletion
· Neutropenia events were mostly transient and manageable
· Infusion‑related reactions were generally low‑grade and did not lead to treatment discontinuation
Looking ahead:
Further follow‑up, as well as data from ongoing first‑line and later‑line studies, will be crucial to understand the durability of remission, optimal timing of intervention, and how best to position ianalumab within the rapidly evolving ITP treatment landscape.
 ”
”
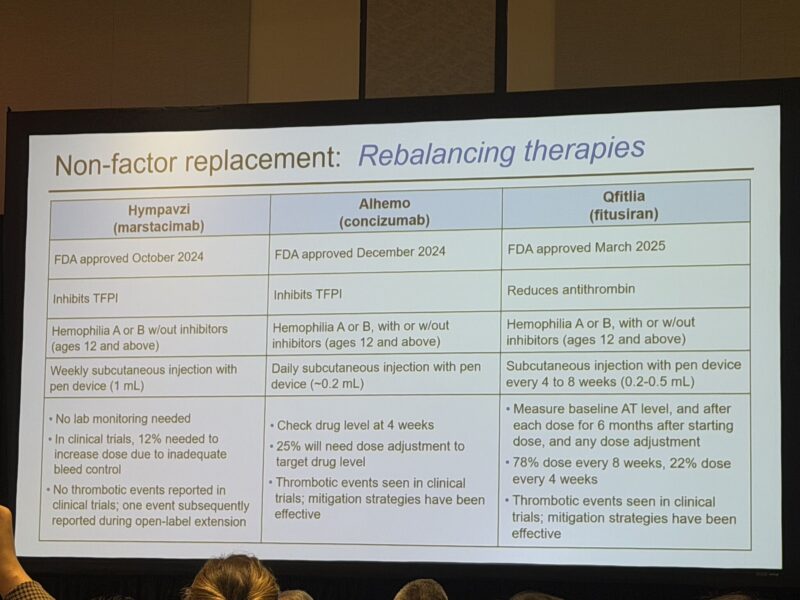
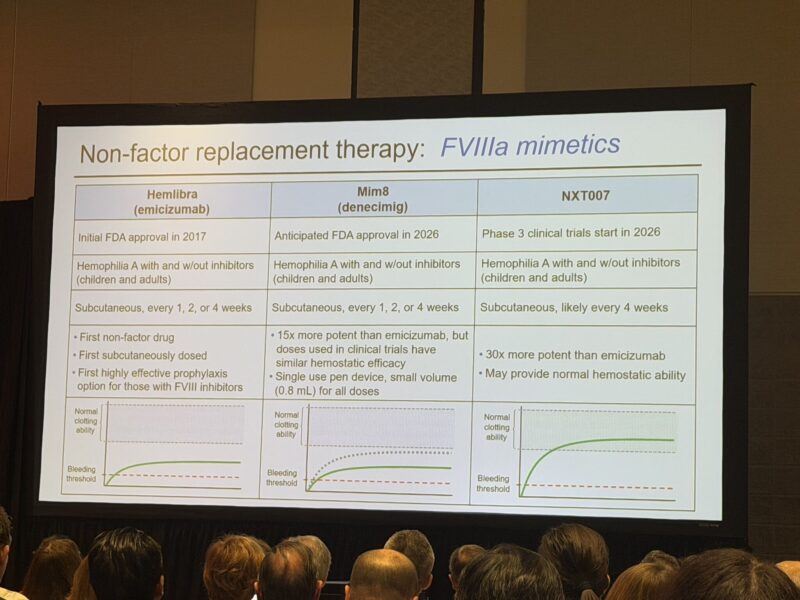
3.”The “Paradox of Choice” is very real in Haemophilia right now.
I just caught Dr. Mark Reding’s insightful presentation at ASH25, and it really highlighted that having more options doesn’t actually make the decision easier.
In fact, it makes it much harder.
We’re looking at a massive toolkit now—extending half-life, bypassing (like the upcoming Mim8 or NXT007), rebalancing (Hympavzi, Alhemo, Qfitlia), or gene therapy (Roctavian, Hemgenix).
But Dr. Reding made a crucial point: the “menu” isn’t actually open to everyone.
You can’t just pick the one with the best marketing. The choice is heavily gated by biology (Type A vs B), age (Rebalancers ≥12y, gene Tx ≥18y), inhibitor Status, distribution and reimbursement issues.
But once you get past those gates, how do you actually choose? Dr. Reding laid out specific factors to break the tie:
- Venous Access is the First Filter: Before debating mechanism, look at the veins. For infants or adults with joint damage, a SubQ option isn’t just a preference—it’s a necessity.
- Risk Aversion is Personal: Some patients want the newest “zero bleed” potency; others prioritize 20 years of safety data. As Dr. Reding noted, both are valid perspectives.
- Activity Level: Bleed risk isn’t static. A high-impact athlete needs a completely different protection profile than someone with a sedentary job.
The tricky part? There is no “winner.”
Because we have zero head-to-head data, we can’t say one is medically better than the other. We’re just trading attributes.
The solution? Realizing that the “best” choice isn’t permanent.
What works for a young children likely won’t be the right fit for an active adult.
The goal isn’t to find the perfect drug for life, but the right fit for right now.
Shared decision-making has never been more important—because we aren’t just prescribing a drug anymore, we’re matching a mechanism to a lifestyle.
 ”
”
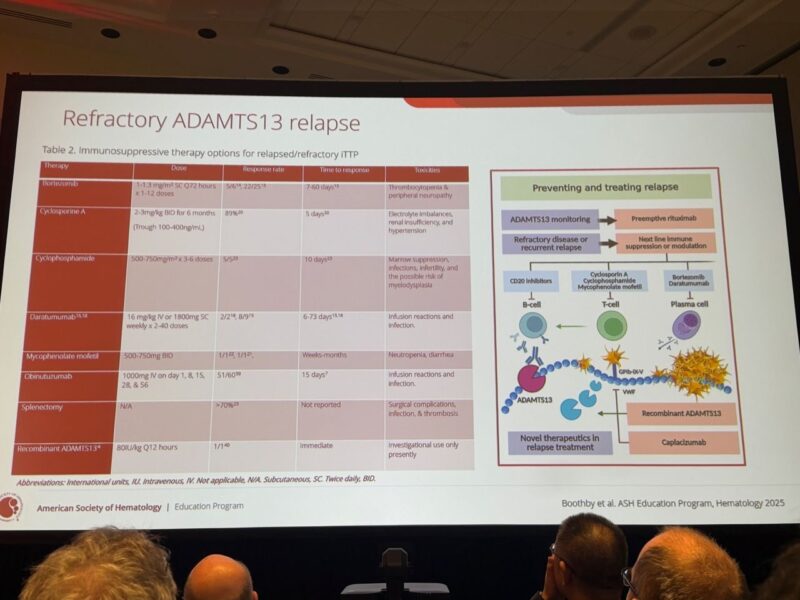
4.”Redefining Long-Term Management in Immune-Mediated TTP.
Just another insightful session at ASH25 by Dr. Senthil Sukumar (Baylor College of Medicine) on the “Challenges and Opportunities in the long-term management of immune-mediated TTP.”
The presentation highlighted a crucial shift from acute crisis management to long-term surveillance and preemptive care.
A few key takeaways that stood out:
Proactive Monitoring is Key:
Regular monitoring of ADAMTS13 activity (every 3–6 months) in clinical remission is essential.
We are moving away from “wait and see” to “predict and prevent.”
The Case for Preemptive Rituximab:
The data presented was compelling regarding the use of off-label preemptive rituximab.
The “French Data” (Jestin et al.): Historical controls with ADAMTS13 <10% had a clinical relapse rate of 74%. Preemptive rituximab reduced this to 15%.
Current Strategy: The proposed algorithm suggests considering preemptive rituximab when ADAMTS13 activity drops below 20%, even without clinical signs of relapse.
Beyond the Blood Counts:
Dr. Sukumar emphasized that remission is not just about platelet counts. Long-term survivors face significant risks, including cardiovascular issues, neurocognitive impairment, and depression.
Refractory Cases and Future Outlook:
For those refractory to rituximab, there might be an future expanding arsenal including proteasome inhibitors (bortezomib), CD38 antibodies (daratumumab), and emerging options like recombinant ADAMTS13.
 ”
”
5.”What matters most – patient reported outcomes at ASH, Cedric Hermans presenting FRONTIER2 data on Mim8 in adolescents and adults with haemophilia A (with and without inhibitors) – and it’s a reminder of what we should have been measuring all along. Not just bleeding control, but how patients actually live.
The numbers:
Treatment burden – 46-62% hit clinically meaningful improvement
Joint pain – 59-95% saw relief depending on where they started
Physical activity – jumped across all groups, 100% in the on-demand cohort
Physical functioning – +4 to +17.6 on the PedsQL scale
But the real story?
88-98% of patients prefer Mim8 over what they were taking before.
Three different PRO measures (Hemo-TEM, PedsQL, JPRS) all pointed the same way.
Better experience, better function, less pain. And it held up over the full 52 weeks.
QW vs QM dosing showed similar PRO benefits – patients actually have flexibility that works.
When you ask patients what matters: It’s your life back.
Less burden. Less pain. More activity.
Those preference numbers speak for themselves.
 ”
”
6.”Maria Elisa Mancuso presented new ASH data on NXT007 prophylaxis in people with haemophilia A (PwHA), with and without FVIII inhibitors – a global, open-label Phase I/II multiple‑ascending dose (MAD) study (first 3 cohorts).
NXT007, a next‑generation FVIII‑mimetic bispecific antibody derived from emicizumab, showed a very consistent picture across pharmacokinetics (PK), pharmacodynamics (PD) and clinical outcomes
Safety/tolerability:
Well tolerated across all dose cohorts, with no thromboembolic events (TEs) / thrombotic microangiopathies (TMAs), no discontinuations due to adverse events (AEs) and D‑dimer levels remaining in the normal range.
Pharmacokinetics:
Dose‑proportional PK with sustained plasma concentrations within the predicted therapeutic range.
Thrombin generation (TG):
Mean TG peak heights in Cohorts 2 and 3 at steady state were comparable to normal‑range FVIII levels.
Bleeding outcomes:
Annualized bleeding rates (ABRs) for treated bleeds markedly decreased after starting NXT007; the majority of participants (≈86%) had zero treated bleeds during the maintenance period. One striking outlier with multiple bleeds had an abnormal vessel in a replaced knee, nicely reminding us that not every bleed signal is “drug failure”.
Immunogenicity:
Most participants developed treatment‑induced anti‑drug antibodies (ADAs) around Day 29, yet these ADAs had no detectable impact on NXT007 PK, efficacy or safety, and showed no cross‑reactivity with emicizumab.
These Phase I/II results strongly support progression of NXT007 into Phase III trials
Guy Young asked an excellent question: If high ADA rates do not translate into altered PK, reduced efficacy or safety concerns, what is the true clinical relevance of routine ADA testing for these bispecifics?
 ”
”
7.”rADAMTS13 Prophylaxis for Congenital Thrombotic Thrombocytopenic Purpura: Promising Results from ASH 2025. Excellent progress in the treatment of congenital TTP (cTTP) was presented yesterday by marie Marie Scully and co-authors at ASH.
The phase 3, prospective, randomized, controlled, open-label study evaluated rADAMTS13 prophylaxis versus FFP across multiple international centers in Austria, France, Germany, Italy, Japan, Poland, Spain, the UK, and the US.
This is the first study to demonstrate that rADAMTS13 can specifically treat cTTP by replacing the missing enzyme in patients with severe hereditary ADAMTS13 deficiency (age 0–70 years).
Over three treatment periods, 47 participants received 1,644 prophylactic infusions—a robust evidence base for this targeted approach:
✓ Zero acute TTP events during rADAMTS13 prophylaxis—significantly lower annualized event rates of isolated TTP manifestations compared to FFP.
Benefit consistent across thrombocytopenia, neurological symptoms, renal dysfunction and other cTTP complications.
✓ Superior patient satisfaction with rADAMTS13 across effectiveness, convenience and overall satisfaction—real-world advantages over regular FFP infusions.
✓ Clean safety profile — no new signals, only one serious adverse event (tachycardia) in periods 1–2, supporting long-term use.
Clinical significance
For people living with cTTP, this represents a paradigm shift from reactive FFP management toward proactive enzyme-replacement prevention.
Reduction in haematologic, neurological and renal TTP manifestations translates to meaningful improvements in quality of life and long-term outcomes.
Huge congratulations to the entire international study group on delivering such important, practice-changing data for the TTP community.
 ”
”
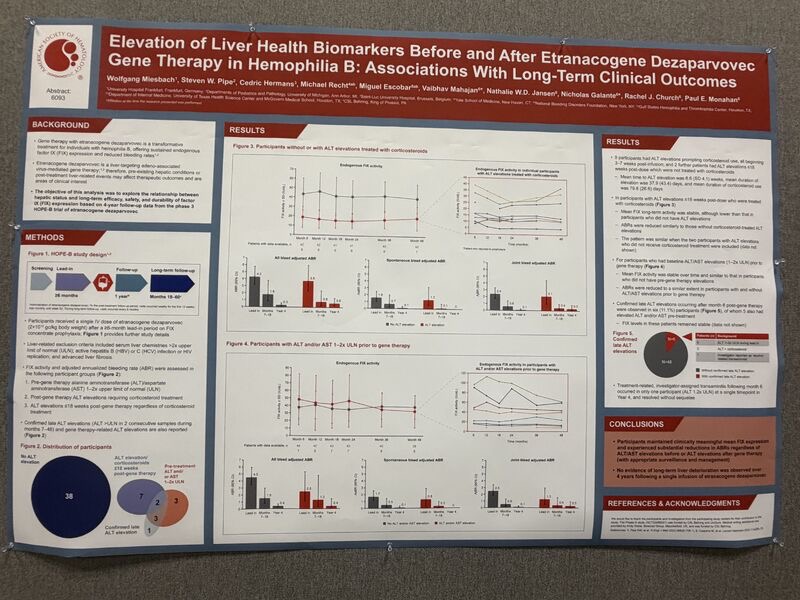
8.”Elevation of Liver Health Biomarkers Before and After Gene Therapy in Haemophilia B. Honored to share our phase 3 HOPE‑B trial data on liver parameters presented at ASH25
Our poster examined if and how ALT/AST elevations before or after gene therapy impact long‑term outcomes in people with haemophilia B treated with etranacogene dezaparvovec — an AAV5‑based vector delivering FIX‑Padua.
- Sustained FIX expression — participants maintained stable, clinically meaningful endogenous FIX activity after managing transaminase elevations with corticosteroids when needed
- Baseline liver abnormalities — individuals with 1–2× ULN ALT/AST at entry achieved comparable FIX levels and bleeding protection to those with normal baseline
- Individual trajectories matter — our data show not just aggregate responses but individual kinetics of FIX recovery and transaminase patterns, revealing heterogeneity in hepatic tolerance
- Robust bleeding control — annualized bleed rates (ABR) remained substantially reduced across all subgroups (including pre‑treatment and post‑treatment elevation cohorts)
No hepatic deterioration — zero evidence of progressive liver disease over 4 years; rare transaminitis episodes resolved without sequelae
Etranacogene dezaparvovec demonstrates durable hepatic safety and FIX durability in haemophilia B, even in patients with mild baseline enzyme abnormalities, with appropriate surveillance and management
Grateful to patients, investigators, coordinators, and collaborators
 ”
”
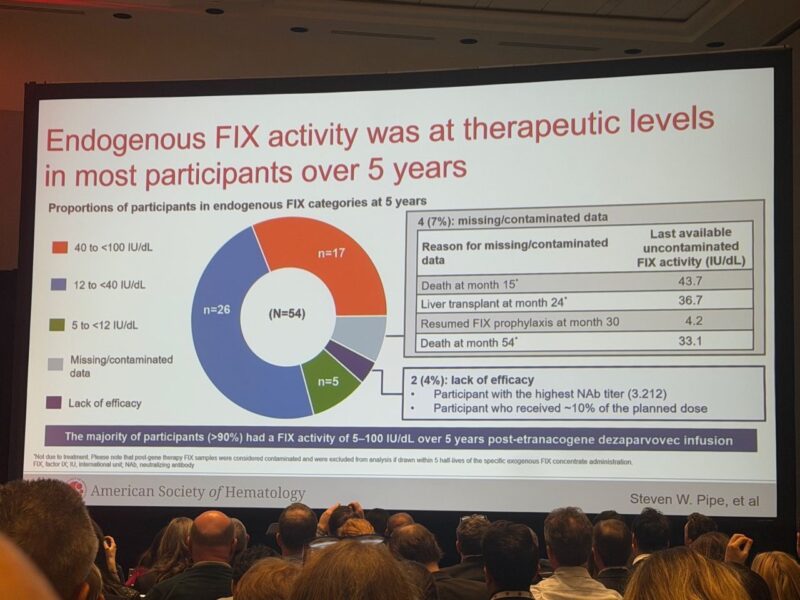
9.”Visualizing 5-year outcomes of haemophilia B gene therapy.
Following up on yesterday’s post about the 5-year HOPE-B results in the New England Journal of Medicine and Steve Pipe’s ASH presentation, here are three key slides that really bring the data to life:
Durable, therapeutic FIX levels at 5 years
Most participants maintained FIX levels in the mild or near-normal range, with >90% between 5–100 IU/dL. The remarkable consistency across the cohort demonstrates sustained gene expression.
Similar FIX expression regardless of pre-existing AAV5 neutralizing antibodies
The boxplots illustrate a pivotal finding: no substantial difference in endogenous FIX activity over time, even in those with pre-existing AAV5 antibodies. This is a game-changer for expanding eligibility in real-world practice – many patients previously thought “ineligible” can now benefit.
Stable FIX activity over the full 5-year follow-up
Mean FIX levels peaked early and then stabilized, remaining in the therapeutic range (mean FIX activity: 39 % and no supraphysiological levels) through Year 5.
What else the full presentation revealed:
- Hepatic safety: Early ALT elevation managed successfully without loss of therapeutic response
- 94% freedom from FIX prophylaxis – transforming daily management into treatment-free living
- 85% reduction in annualized joint bleeding rate – from 2.34 to 0.35
- Zero FIX inhibitors | Zero thrombotic events
Deep gratitude to Steve Pipe, the HOPE-B investigators, CSL, and above all the 54 participants whose courage and commitment made these landmark 5-year data possible.
 ”
”
10.”ASH ITP Guidelines 2025: Open for Comments
After years of rigorous evidence review using GRADE methodology, the ASH ITP guidelines panel—17 diverse international experts—has reimagined how we manage adult ITP.
Great presentation by Keith McCrae and colleagues at the ASH Kongress.
We’re moving from corticosteroid monotherapy to combination therapy from day one.
The 2025 guidelines introduce this option (completely absent in the 2019 guidelines) recommending either rituximab + corticosteroids OR TPO-RA + corticosteroids (± IVIG) rather than steroids alone.
This directly contradicts 2019’s recommendation, which explicitly favored steroid monotherapy—a recommendation that is not being carried forward.
Specific sequencing guidance for treatment-resistant ITP: splenectomy OR TPO-RA (conditional), rituximab over splenectomy (conditional), and TPO-RA over rituximab (conditional).
Result: Earlier, targeted immunomodulation. Less prolonged steroid exposure. More patient options.
Pediatric recommendations remain unchanged, showing the panel’s discipline in focusing only where new evidence demands change.
Public comment open until December 12th, 2025.
Shape the final recommendations at [email protected]
 ”
”
11.”iTTP remission ≠ cure. The vascular injury persists.
TTP as a cardiovascular disease equivalent.
Just finished Dr. Senthil Sukumar‘s ASH25 session on long-term iTTP management.
This completely changed how I think about these patients.
The Numbers Hit Different:
- 28.6% of survivors → major cardiovascular events
- 50% → silent cerebral infarcts (vs 16.6% controls)
- Cardiovascular complications 10–20 years EARLIER
- 80% depression | 35% post traumatic stress disorder
- >60% measurable neurocognitive impairment
- 71% moderate-severe headaches
- 20% can’t work due to complications
The Game Changer: Endothelial Damage That Doesn’t Heal
Patients are not “fine” after remission. The endothelium stays injured.
Stress cardiac MRI shows reduced perfusion + impaired coronary vasodilation—the heart can’t increase blood flow under stress even when ADAMTS13 normalizes.
The Actionable Part
ADAMTS13 ≤70% = 27.6% stroke rate
ADAMTS13 >70% = ZERO ischemic strokes
As a consequence, treat iTTP as CVD Equivalent
- Comprehensive cardiovascular risk factor optimization (lipids, A1C, smoking, BP, weight, activity)
- Baseline mood + neurocognitive screening for ALL survivors
- Neuropsych testing every 1–2 years
- Multidisciplinary teams (neuro, psych, cardio)
 ”
”
12.”Beyond Immunosuppression: Two new ways to treat ITP. David Kuter gave a great presentation at ASH 2025:
- Increase Platelet Production
The latest TPO receptor agonist, hetrombopag, now in Phase 3 trials, offers a direct pathway to boost platelet synthesis—moving away from suppression toward restoration. - Decrease Platelet Destruction
A diverse arsenal of targeted therapies can redefineour strategy:
– Anti-CD38 antibodies (daratumumab, mezagitamab)
– BAFF/BAFF-R inhibitors
– FcRn inhibitors (efgartigimod)
– BTK inhibitors (rilzabrutinib, orelabrutinib)
– Complement inhibitors + immunomodulatory approaches
– T-cell engagers and CAR-T therapies
ITP is now recognized as a disorder of both reduced production AND increased destruction—this understanding drives treatment selection toward complete remission, not just disease suppression.
 ”
”
All from ASH25 featured in Hemostasis Today.
-
Jan 26, 2026, 05:09Wathsala Manindrani Gives a A Practical Perspective on Red Cell Exchange Transfusion
-
Jan 26, 2026, 04:59Abdul Mannan: BDUC – 4 Letters That Make Many Haematologists Uncomfortable
-
Jan 26, 2026, 04:51Manoj Kumar Singh: Power In Me Foundation Celebrates 2026 as Year Of Rare
-
Jan 26, 2026, 04:40Heghine Khachatryan: Did You Know VWD Comprises 3 Main Types?
-
Jan 25, 2026, 15:57Céline Chapelle Shares Clinical Predictors From the API-CAT Trial
-
Jan 25, 2026, 15:42Francesco Lo Monaco on Heart Disease Starting Quiet While Your Labs Speak First
-
Jan 25, 2026, 15:25Muhammad Ibrahim on Efficacy and Safety of Extended DOACs Use in VTE
-
Jan 25, 2026, 15:08Tushar Pandey on Managing Thrombotic Thrombocytopenic Purpura
-
Jan 25, 2026, 14:55Carolina Contreras Cuevas Shares a Nationwide Study on VTE in PAD
