
New Insights On Pre-Existing AAV5 Abs in Haemophilia B Gene Therapy
Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital, shared on LinkedIn:
”Exited to share our new paper: New insights on pre-existing AAV5 antibodies in haemophilia B gene therapy!
First comprehensive longitudinal analysis of AAV5 NAb stability over 6+ months in haemophilia B patients (median 8 months of follow-up).
Unlike most gene therapy trials that exclude NAb+ patients, HOPE-B (etranacogene dezaparvovec) enrolled participants regardless of pre-existing AAV5 NAb titers.
Study Highlights:
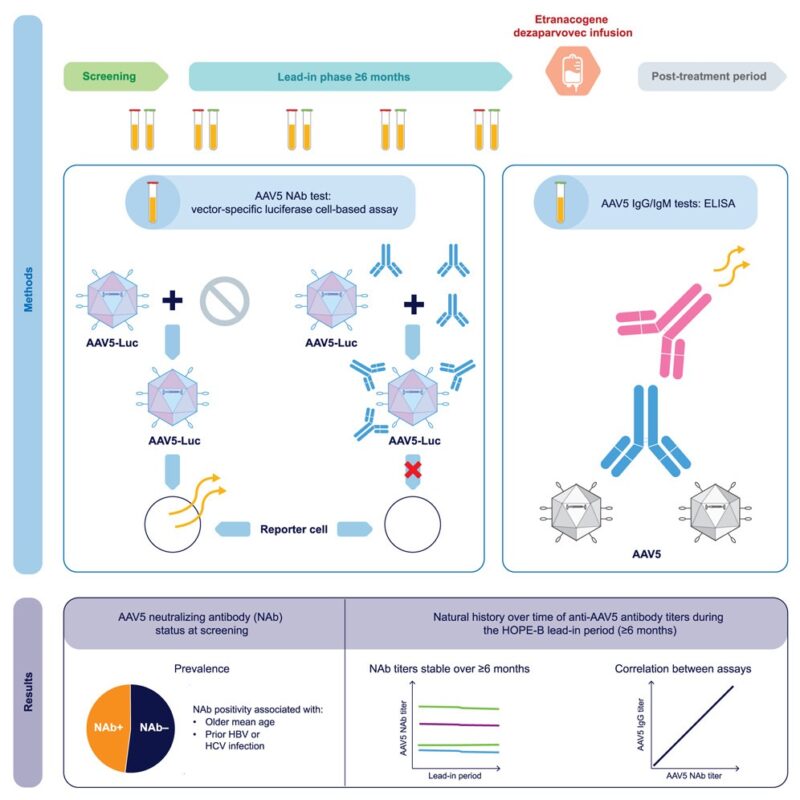
– 67 adult males with moderate-to-severe haemophilia B followed over median 8 months (240 days)
– 47.8% had pre-existing AAV5 neutralizing antibodies (NAbs) at screening – median titer was 58
– Comprehensive analysis of both neutralizing antibodies (NAbs) and binding antibodies (IgG/IgM)
Clinical Insights:
- AAV5 NAb titers remain stable over 6+ months – median intra-patient coefficient variation only 25%
- Strong correlation between NAb and IgG binding antibodies (r=0.96) across multiple timepoints
- Age association: NAb positivity more frequent in patients ≥50 years, particularly those with prior hepatitis B/C exposure
Seroconversion Patterns:
NAb- to NAb+ Seroconversion:
Very rare: Only ~5% of participants (2 out of 67) seroconverted from NAb- to NAb+ during the lead-in period.
One clear case showed seroconversion at 4 months with contemporaneous transient IgM increase, consistent with new AAV exposure
NAb+ to NAb- Seroreversion:
More common: Approximately 10% of participants seroreverted from NAb+ to NAb-.
All participants who seroreverted had NAb titers ≤25 at screening, close to assay detection limits
Clinical Implications:
– Screening flexibility: NAb testing can be done several months before planned gene therapy without concern for significant titer changes
– Patient selection confidence: Provides robust data supporting inclusion of NAb-positive patients in etranacogene dezaparvovec treatment
Remaining Challenges:
– Different cell types, reporter systems, and MOI ratios across laboratories
– Variable calculation methods for titer determination
– No human anti-AAV calibrators for absolute quantification
Congratulations to Robert Klamroth and co-authors and thank you to CSL for supporting this study.”
Read the full article here.
Article: Natural history of preexisting AAV5 antibodies in adults with hemophilia B during the lead-in of the etranacogene dezaparvovec phase 3 study
Authors: Robert Klamroth, Michael Recht, Nigel S. Key, Wolfgang Miesbach, Steven W. Pipe, Radoslaw Kaczmarek, Douglass Drelich, Blanca Salazar, Sandra Le Quellec, Paul E. Monahan, Nicholas Galante, Paul van der Valk, Jacqueline Tarrant
Stay updated on all scientific advances in the field of hemophilia with Hemostasis Today.
-
Feb 22, 2026, 07:20Leonardo Cardoso: Mechanical Thrombectomy Improves Functional Independence, Outcomes and Reduces Mortality
-
Feb 22, 2026, 07:08Ashkan Shoamanesh: OCEANIC-STROKE Focus on Stroke Subtypes and Response to Asundexian at ISC26
-
Feb 22, 2026, 06:43Aurelio Maggio: Is It Time to Align Thalassemia Biology with Regulatory Science?
-
Feb 22, 2026, 06:32Priyansh Shah: Integrating Food-Based Nutrition Interventions Into Health Care Delivery
-
Feb 22, 2026, 06:18Thomas Reiser: How Can Medical and Healthcare Associations Stay Impactful in a Changing Environment?
-
Feb 22, 2026, 05:58Mark Crowther: Why Leaders Must Model What They Expect
-
Feb 21, 2026, 15:58Addressing Underdiagnosis and Care Inequities in Women and Girls with Bleeding Disorders – WFH
-
Feb 21, 2026, 15:49Sheharyar Raza: The Leaky Pipeline of AI and Machine Learning in Transfusion Medicine
-
Feb 21, 2026, 15:21Joanna Sadowska: Watching a Stem Cell Divide Reveals Life’s Blueprint

