
Lucia Masarova/X
Sep 5, 2025, 12:18
Lucia Masarova Presents Results of Phase III SURPASS-ET Trial at SOHO 2025: Ropeg’s Efficacy for Essential Thrombocythemia
MPN Hub shared on X:
”CONGRESS | SOHO 2025 | POSTER
Lucia Masarova, MD Anderson Cancer Center
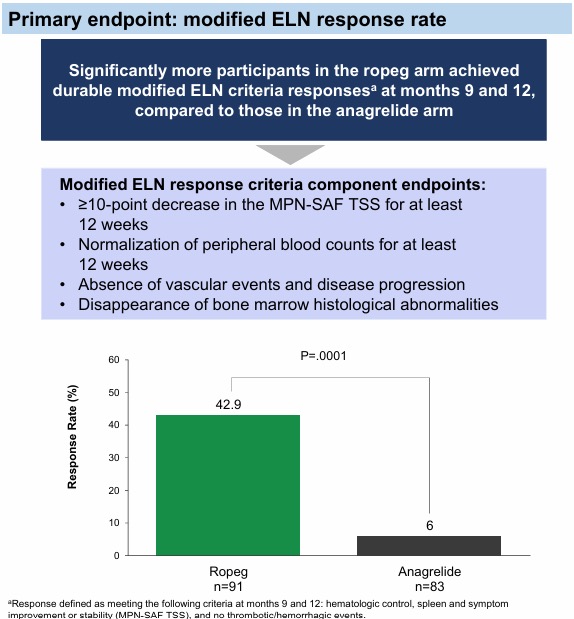
Presents results from the phase III SURPASS-ET trial of ropeginterferon alfa-2b (n=91) vs anagrelide (n=83) in patients with HR Essential Thrombocythemia who are resistant or intolerant to hydroxyurea.
Ropeg showed superior efficacy over anagrelide (RR, 42.9% vs 6.0%, P=0.0001) and demonstrated a favorable safety profile.”
All on Hemostasis and Thrombosis from SOHO 2025 featured in Hemostasis Today.
-
Jan 22, 2026, 15:36We Must Roll Up Our Sleeves And Help: José Antonio García Erce on Plasma Donation
-
Jan 22, 2026, 15:25Nita Radhakrishnan on Challenges In Congenital Afibrinogenemia
-
Jan 22, 2026, 15:10Jin Q Gives a Summary of 2025’s Most Impactful Cell and Gene Therapy Milestones
-
Jan 22, 2026, 14:57Nirav Dhanesha on CD14 Acting As A Functional Driver of DVT
-
Jan 22, 2026, 11:41Jamilla Goedegebuur and Colleagues on VTE Management in Case of PAD
-
Jan 22, 2026, 11:28Abdulrahman Katib on API-CAT Trial’s Evaluation of Apixaban Dosing
-
Jan 22, 2026, 11:19Bruno Odisio: Ablation Margins Are Software-Dependent
-
Jan 22, 2026, 10:38Pedro Perez: The VTE Market Is Clearly Entering Its Next Phase
-
Jan 22, 2026, 10:29Marvin Garcia Reyes Presents a Case of Aorto-Visceral and Aorto-Iliac Thrombosis
