
Ahmed Bennis on Use of Evolocumab in Patients without a Previous MI or Stroke
Ahmed Bennis, Professor of Cardiology at Ibn Rochd University Hospital, shared on LinkedIn:
”First favorite
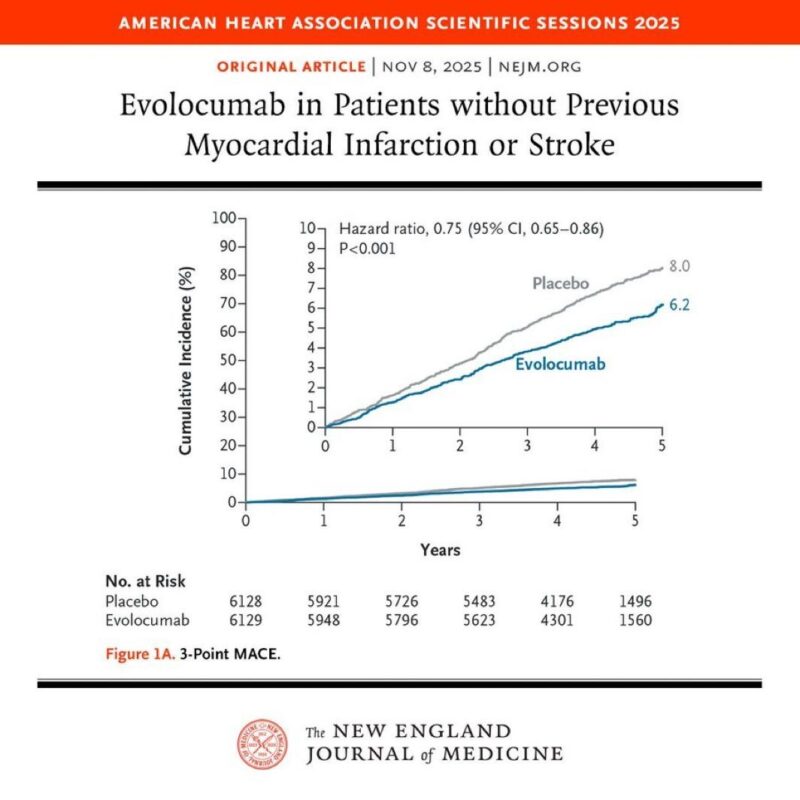
In patients without a previous heart attack or stroke, evolocumab reduced major cardiovascular events by 25% and lowered LDL cholesterol by approximately 55% compared to placebo.
These benefits were achieved without a significant increase in adverse events.
Evolocumab in Patients without a Previous Myocardial Infarction or Stroke
Evolocumab, a proprotein convertase subtilisin/kexin type 9 (PCSK9), reduces the risk of major adverse cardiovascular events (MACE) in patients with a history of myocardial infarction, stroke, or symptomatic peripheral artery disease. The effect of evolocumab on the risk of MACE in patients without a history of myocardial infarction or stroke is unknown.
METHODS
We conducted an international, randomized, double-blind, placebo-controlled trial evaluating evolocumab in patients with atherosclerosis or diabetes, without a history of myocardial infarction or stroke, and with LDL cholesterol levels of at least 90 mg/dL.
Patients were randomized in a 1:1 ratio to receive either evolocumab 140 mg every two weeks or placebo.
The two primary endpoints were a composite endpoint of death from coronary heart disease, myocardial infarction, or ischemic stroke (3-point MACE) and a composite endpoint of 3-point MACE or ischemia-driven arterial revascularization (4-point MACE).
RESULTS
A total of 12,257 patients were randomized to receive either evolocumab (6,129 patients) or placebo (6,128) and were included in the efficacy analyses.
The median age of patients was 66 years, 43% were women, and 93% were white.
The median follow-up duration was 4.6 years.
A 3-point major adverse cardiovascular event (MACE) occurred in 336 patients (Kaplan-Meier estimate at 5 years: 6.2%) in the evolocumab group, compared with 443 (8.0%) in the placebo group (hazard ratio: 0.75; 95% confidence interval [CI]: 0.65 to 0.86; p < 0.001).
Grade 4 major adverse cardiovascular events (MACE) occurred in 747 patients (5-year Kaplan-Meier estimate: 13.4%) in the evolocumab group, compared with 907 (16.2%) in the placebo group (hazard ratio: 0.81; 95% CI: 0.73 to 0.89; p < 0.001).
No significant differences were observed between the groups in the incidence of adverse events.
CONCLUSIONS
PCSK9 inhibition with evolocumab resulted in a lower risk of first cardiovascular event than placebo in patients with atherosclerosis or diabetes and no history of myocardial infarction or stroke.”
Read the full article in NEJM.
Article: Evolocumab in Patients without a Previous Myocardial Infarction or Stroke
Authors: Erin A. Bohula, Nicholas A. Marston, Ajay K. Bhatia, Gaetano M. De Ferrari, Lawrence A. Leiter, Jose C. Nicolau, Jeong-Gun Park, Julia F. Kuder, Sabina A. Murphy, Emileigh Walsh, Huei Wang, Vladimir Blaha, Andrzej Budaj, Jan H. Cornel, Assen Goudev, Robert Gabor Kiss, Alberto J. Lorenzatti, Alexander Parkhomenko, Marcoli Cyrille, Gabriel Paiva da Silva Lima, E. Magnus Ohman, Robert P. Giugliano, Marc S. Sabatine
Stay updated on all scientific advances with Hemostasis Today.
-
Jan 8, 2026, 05:51Dominika Jędrzejewska Akbaş Links Sleep Deprivation, Obesity, and Stroke
-
Jan 8, 2026, 05:42Rob Maloney: A Bruise that Did Not Go Away…
-
Jan 8, 2026, 05:33Matías J Alet on Treating Early Neurological Deterioration in Lacunar Stroke
-
Jan 8, 2026, 05:11Thomas Meinel Shares The English Stroke Guidelines 2026
-
Jan 8, 2026, 05:03Hilla Ben-Pazi: We Were Pleased to Receive a Grant from the Israeli Ministry of Economy
-
Jan 8, 2026, 04:24Nikhil Agrawal Breaks Down Perioperative Anticoagulation
-
Jan 8, 2026, 03:58Louise St Germain Bannon Appointed Interim Executive Director of ISTH
-
Jan 8, 2026, 03:47Wolfgang Miesbach on Fernando Corrales-Medina’s Team’s Latest Cross-Sectional Study on Haemophilia
-
Jan 8, 2026, 03:28Rick Matthews: Powerful Perspective from Erin and the NBCA Team
