
Nikhil Agrawal Breaks Down Perioperative Anticoagulation
Nikhil Agrawal, Internal Medicine Specialist (MBBS) at Safdarjung Hospital, shared a thread on X:
”Perioperative Anticoagulation
This is one of the hardest decisions in medicine.
Stopping too early increases clots.
Stopping too late increases bleeding.
This thread simplifies everything.
Start with the fundamental question
Perioperative AC is about preventing two dangers
• Surgical bleeding
• Thrombosis when AC is stopped
Every patient needs BOTH risks assessed before making any move.
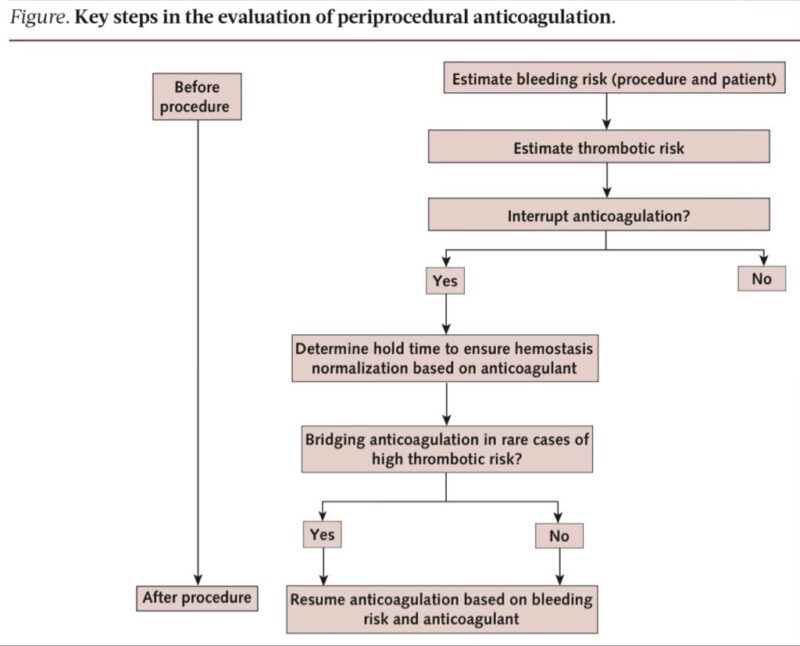
Step 1. Classify the procedure bleeding risk
Three levels guide the entire plan
• Minimal
• Low to moderate
• High
This determines whether AC is continued, temporarily held, or fully reversed.
Minimal bleeding risk (AC usually continued)
Examples
• Dental procedures
• Minor dermatology
• Pacemaker implantation
• Cataract surgery
• Arthrocentesis
• Diagnostic endoscopy
These have extremely low procedure-related bleeding even if AC is continued.
Low to moderate bleeding risk (AC usually interrupted)
Common examples
• Laparoscopic cholecystectomy
• Hernia repair
• Most abdominal and thoracic surgeries
• Coronary angiography
These procedures typically need short DOAC hold or warfarin interruption.
High bleeding risk procedures (strict AC control needed)
Examples
• Neurosurgery
• Major vascular surgeries
• Major cancer surgeries
• Total hip or knee replacement
• Surgeries with neuraxial anesthesia
These require near-complete anticoagulant clearance and controlled restart.
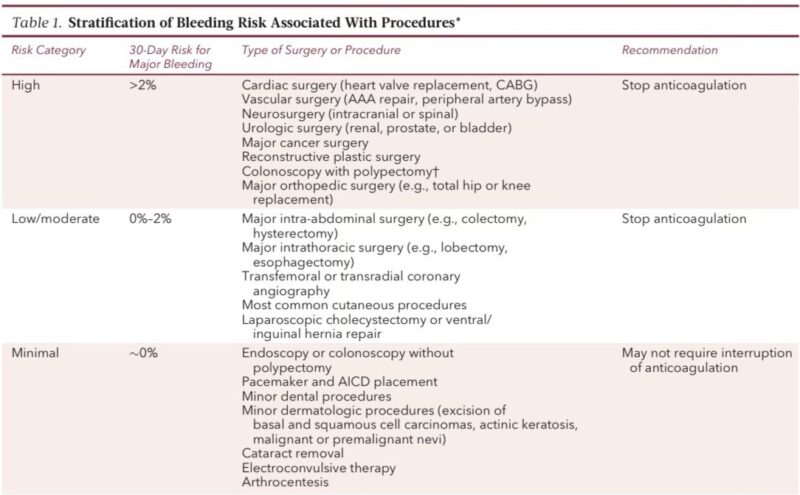
Step 2. Assess thrombosis risk
You must stratify thrombosis risk from
• Mechanical valves
• Atrial fibrillation
• VTE
This step decides whether interruption is safe and whether bridging is required.
Mechanical valve patients equals highest risk group
High-risk features
• Mitral mechanical valve
• Caged-ball or tilting-disk valves
• Valve placed <3 months
• Prior stroke
• AF or LV dysfunction
Mitral mechanical valves have up to 22 percent annual stroke risk without AC.
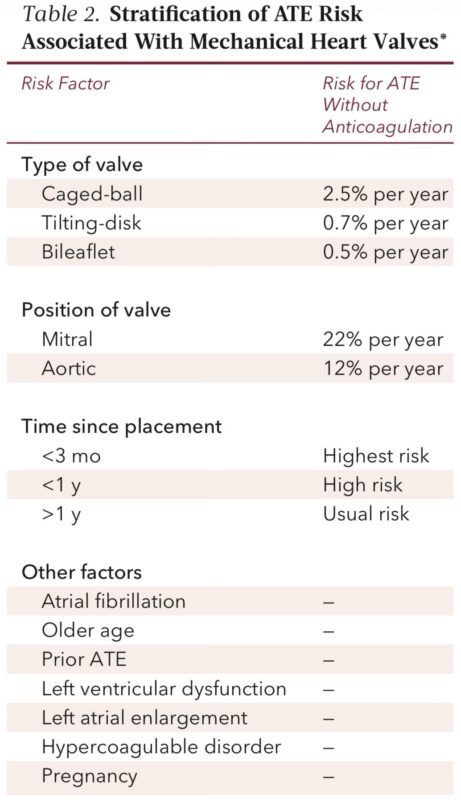
Atrial fibrillation thrombosis risk (CHA2DS2-VASc)
Risk levels
• 0 to 3 → low
• 4 to 6 → moderate
• ≥7 or recent stroke → high
BRIDGE trial: low and moderate AF patients bleed more if bridged without any reduction in stroke.
VTE thrombosis risk depends mainly on timing
• VTE <3 months → high risk
• VTE 3 to 12 months → moderate
• VTE >12 months → low
Strong thrombophilias (APS, protein C/S, AT deficiency) push the patient into high-risk category irrespective of timing.
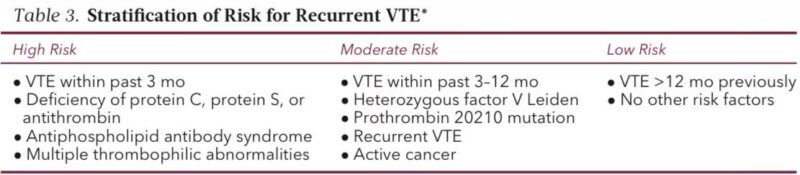
Step 3. Decide if AC must be interrupted
Simple rule
• Minimal bleeding risk → continue AC
• Low/moderate → interrupt safely
• High bleeding risk → interrupt and ensure INR normalization or full DOAC washout before incision
This step prevents intraoperative bleeding catastrophes.
Step 4. Bridging decisions (for warfarin only)
Bridging equals giving LMWH or UFH during warfarin interruption.
Modern evidence: bridging should be used only in very high thrombosis risk patients because it increases bleeding without reducing thrombosis in most others.
(PERIOP2 + BRIDGE trials)
Who truly needs bridging (rare but crucial)
Use bridging only when stopping AC is extremely unsafe:
• Mechanical mitral valve
• Caged-ball or tilting-disk valve
• CHA2DS2-VASc ≥7
• Recent stroke or TIA
• VTE <3 months
• APS or multiple thrombophilias
These are the ONLY groups where bridging has a favorable risk–benefit ratio.
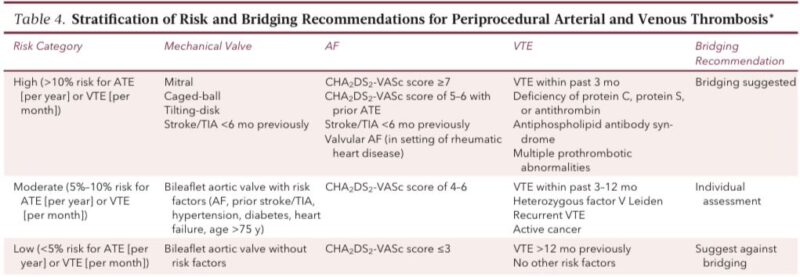
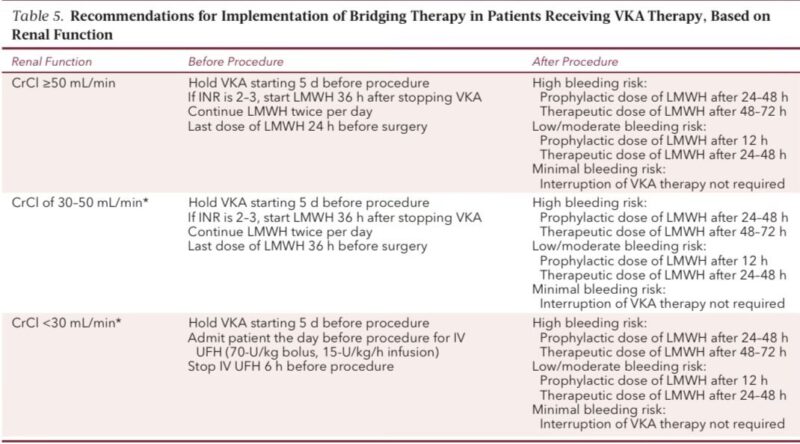
Warfarin perioperative plan (clear and detailed)
Stopping
• Stop 5 days before surgery
• Check INR 7–10 days before, then again night before
• If INR >1.5 → give 1.25–2.5 mg vitamin K
Restarting
• Low/mod risk → restart 12 hours post-op
• High bleed risk → restart 24–36 hours post-op
Bridging ONLY if patient is very high thrombosis risk.

Bridging protocol (if indicated)
• Start LMWH 36 hours after last warfarin dose
• Last LMWH dose 24 hours before procedure
• If CrCl <30 → use UFH instead
• Stop UFH 6 hours before incision
Restart after surgery only when hemostasis is secure.
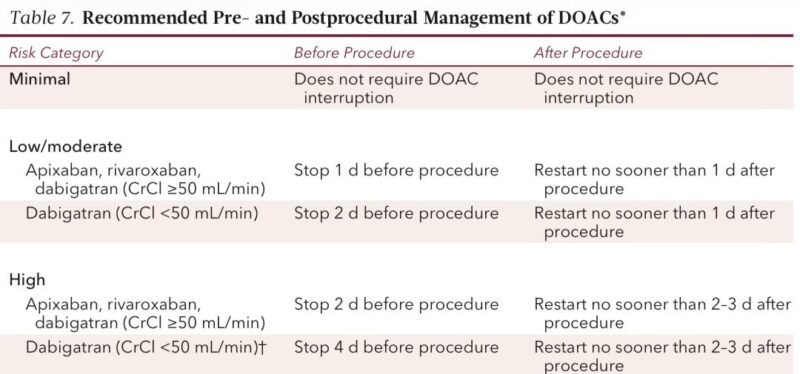
DOAC perioperative plan (simple, precise, evidence-backed)
Low/mod bleed risk
• Hold apixaban/rivaroxaban 1 day before
• Dabigatran: 1 day if CrCl ≥50, 2 days if <50
High bleed risk
• Hold apixaban/rivaroxaban 2 days
• Dabigatran: 2 days if CrCl ≥50, 4 days if <50
This is directly from the PAUSE protocol with very low bleeding/thrombotic rates.
Emergency surgery (need reversal)
If there’s no time for natural clearance:
Warfarin
• Vitamin K
• PCC preferred over FFP
DOACs
• Dabigatran → Idarucizumab
• Factor Xa inhibitors → Andexanet Alfa or PCC
Note: Andexanet may cause thrombosis and DOAC rebound.”
Stay updated with Hemostasis Today.
-
Feb 23, 2026, 18:13Fight4Hematology Supports Research and Empowers the Next Generation – ASH
-
Feb 23, 2026, 17:59Wolfgang Miesbach: Real-World Evidence of Emicizumab on Joint Outcomes in Hemophilia A
-
Feb 23, 2026, 17:56Shiny K Kajal: The Transfusion Reaction We Often Miss
-
Feb 23, 2026, 17:53Radheshyam Meher: Contributing to the Transfusion Evidence Round-Up for International Childhood Cancer Day 2026
-
Feb 23, 2026, 17:46Mahesan Subramaniam: The Physiological Impact of Anger on Immunity
-
Feb 23, 2026, 17:42Bryan Fry: First Evidence That Bothrops atrox Venom Directly Activates Human Factor VII
-
Feb 23, 2026, 17:34Bastu Odoka: Why Blood Should NOT be Left at the Bedside to ‘Warm’
-
Feb 23, 2026, 17:28Henry Burkitt: Patients Are Challenging How the Medicines Policy System Works in England
-
Feb 23, 2026, 16:50Mutaz Al‑Sabah: Interesting Webinar on FH in Women is Now Available to Watch

