VAYHIT2 Trial: Ianalumab’s Role in Enhancing ITP Treatment Outcomes
VAYHIT2 trial evaluated the combination of ianalumab and eltrombopag in patients with primary immune thrombocytopenia (ITP) who had previously been treated with corticosteroids. The trial demonstrated that ianalumab, when paired with eltrombopag, significantly improved the time to treatment failure (TTF), marking the primary endpoint. Ianalumab, a novel monoclonal antibody, is also being investigated in other B-cell driven autoimmune diseases.
Ianalumab has been granted Orphan Drug Designation by both the FDA and the European Medicines Agency (EMA) for ITP treatment.
Understanding the Mechanisms of Action of Ianalumab and Eltrombopag
Ianalumab (VAY736)

Ianalumab, developed by Novartis, is a fully human monoclonal antibody that targets B cells by binding to the BAFF-R (B-cell activating factor receptor). By blocking the BAFF-R receptor, ianalumab interrupts signals that are critical for B cell survival and function, leading to the depletion of B cells. This action is important because B cells play a key role in the pathogenesis of several autoimmune diseases, including Sjögren’s disease, systemic lupus erythematosus (SLE), and immune thrombocytopenia (ITP).
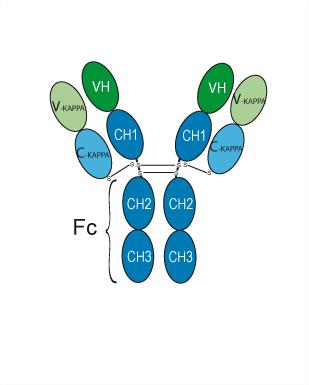
Additionally, ianalumab induces ADCC, a mechanism where immune cells like natural killer (NK) cells and macrophages are recruited to destroy B cells. This helps eliminate overactive or dysfunctional B cells that contribute to autoimmune responses.
By targeting B cells and modulating immune responses, ianalumab aims to provide durable clinical responses and reduce long-term reliance on immunosuppressive therapies. This could be beneficial in autoimmune diseases like ITP, where the immune system mistakenly destroys platelets.
Eltrombopag
Eltrombopag is an oral medication that acts as a thrombopoietin receptor agonist. It binds to the thrombopoietin receptor (TpoR) on megakaryocytes (platelet-producing cells in the bone marrow) and hematopoietic stem cells, stimulating their proliferation and differentiation. This promotes platelet production in the bone marrow.
Mechanism of Platelet Production: By binding to the TpoR, eltrombopag mimics the action of thrombopoietin, the natural hormone that regulates platelet production. This action leads to an increase in platelet count, helping to address the thrombocytopenia (low platelet count) seen in conditions like ITP.
Eltrombopag is primarily used to treat chronic ITP and other thrombocytopenic conditions where platelet production is insufficient. It helps reduce the need for platelet transfusions and can be used in patients who have failed other treatments, including corticosteroids and IVIG (intravenous immunoglobulin).

“While current treatments for ITP are generally effective in raising platelet counts, many patients require life-long treatment to maintain safe levels, which can create a lasting treatment burden, The results from VAYHIT2 are encouraging, as they suggest that ianalumab may support longer periods of disease control and reduce the need for continuous treatment”
said Adam Cuker, M.D., Professor of Medicine and Chief, Section of Hematology, University of Pennsylvania.

“For many people living with ITP, chronic treatment can disrupt their daily life due to the burden of regular dosing, dose adjustments and side effects. These positive top-line results from the Phase III study highlight the potential of ianalumab, if approved, to deliver long-term disease control with four once-monthly doses and enable extended time off treatment.”
said Shreeram Aradhye, M.D., President, Development and Chief Medical Officer, Novartis.
Crash course about Immune Thrombocytopenia
Immune thrombocytopenia (ITP), formerly known as idiopathic thrombocytopenic purpura, is an autoimmune disorder in which the immune system produces autoantibodies against platelets, leading to thrombocytopenia (low platelet count), purpura (bruising), and hemorrhagic episodes (bleeding). ITP can be categorized into primary (without any underlying disease) and secondary (caused by other factors like drugs or autoimmune conditions such as lupus).
The disorder is particularly common in children, often leading to spontaneous remission. In adults, remission is rare, and splenectomy (surgical removal of the spleen) is commonly required after trying other medical treatments.
Pathophysiology
The development of ITP is primarily driven by autoantibodies, mostly IgG, that target platelet membrane proteins like GP IIb/IIIa, Ib/IIa, and VI. These autoantibodies bind to platelets, marking them for destruction by macrophages, particularly in the spleen, thus reducing the platelet count. T-cell-mediated cytotoxicity may also contribute, affecting the megakaryocytes in the bone marrow that produce platelets.
Primary ITP occurs without any underlying cause, while secondary ITP is typically triggered by:
- Infections like HIV, hepatitis C, cytomegalovirus, and varicella-zoster virus.
- Medications, such as phenytoin, heparin, quinine, and vancomycin.
- Autoimmune conditions like systemic lupus erythematosus (SLE) and Evans syndrome.
- Malignancies such as chronic lymphocytic leukemia (CLL).
- Endocrine disorders like hypothyroidism and Addison disease.
Clinical Presentation
Common symptoms include mucocutaneous bleeding (e.g., petechiae, ecchymosis, epistaxis), fatigue, and heavy menstrual bleeding in women.
Severe cases may involve life-threatening bleeding, including intracranial hemorrhage (ICH), which is the most feared complication.
Diagnosis
- CBC typically reveals thrombocytopenia (platelet count <100,000/μL) with normal WBC and hemoglobin levels.
- Additional tests may include bone marrow biopsy (if malignancy or bone marrow failure is suspected) and autoantibody testing (though platelet autoantibody tests have low sensitivity for ITP).
Management
First-Line Treatment- Corticosteroids (e.g., prednisone) are commonly used to treat newly diagnosed ITP with platelet counts <30,000/μL. For children with minor symptoms, observation may be appropriate.
Second-Line Treatment- If first-line therapy is ineffective, thrombopoietin receptor agonists (TPO-RAs) such as eltrombopag or romiplostim are used. Rituximab, an immunosuppressive monoclonal antibody, is another option, especially for chronic or refractory ITP.
Splenectomy is typically reserved for chronic or refractory ITP after medical treatments fail.
Emerging Therapies- New treatments like fostamatinib, efgartigimod, and rilzabrutinib are under investigation to manage ITP more effectively.
About VAYHIT2 trial
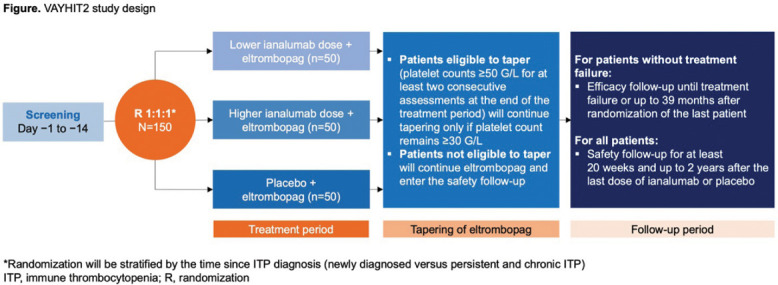
VAYHIT2 (NCT05653219): A Phase III, multi-center, randomized, double-blind study aimed at evaluating ianalumab’s efficacy in combination with eltrombopag in adult patients with ITP who had failed prior corticosteroid treatment.
Study Design
- Patients were randomly assigned to receive either ianalumab (3 mg/kg or 9 mg/kg) or placebo, along with eltrombopag.
- The primary endpoint was time to treatment failure (TTF), defined by several factors such as platelet count below 30 G/L after 8 weeks, the need for rescue therapy, or initiation of new ITP treatments.
- Secondary endpoints included the rate of sustained improvements in platelet counts, and quality of life and fatigue assessments.
Results
The VAYHIT2 trial results suggest that ianalumab, when used alongside eltrombopag, may offer significant benefits for ITP patients by providing longer periods of disease control and potentially reducing the need for long-term treatments. These findings are expected to contribute to future regulatory submissions and further investigations in ITP and other B cell-driven diseases.
- Ianalumab plus eltrombopag showed a significant improvement in TTF compared to placebo plus eltrombopag.
- The combination therapy also resulted in a higher rate of sustained platelet count improvements at six months, meeting the key secondary endpoint.
- The safety profile of ianalumab was consistent with previous studies, with no new safety signals observed.
Path Forward and Key Milestones
Ongoing Studies: The VAYHIT1 trial (for first-line ITP) and additional Phase III trials for other diseases will provide further insights into ianalumab’s broad applicability.
Regulatory Submission: The results will be submitted for regulatory review in 2027, including data from the ongoing first-line ITP study, VAYHIT1.
Conclusion: A New Era for ITP Treatment
The results of the VAYHIT2 Phase III trial indicate that ianalumab in combination with eltrombopag provides significant improvements in time to treatment failure and sustained platelet count in patients with ITP, potentially reducing the need for long-term treatments. These promising findings highlight the potential of ianalumab as a novel therapy for ITP and other B cell-driven autoimmune diseases, with further regulatory submissions and ongoing trials expected to shape its future in clinical practice.
Written by Sona Karamyan, MD
-
Jan 9, 2026, 09:25Emma Groarke Shares A Comprehensive Review on VEXAS Syndrome
-
Jan 9, 2026, 09:13Anirban Sen Gupta Presents PlateChek
-
Jan 9, 2026, 08:58Oscar Pena Shares KDIGO 2026 Update: 5 Critical Changes for Anemia in CKD Management
-
Jan 9, 2026, 07:38Bartosz Hudzik on Subacute Coronary Occlusions: Navigating the Gray Zone in CAD Care
-
Jan 9, 2026, 07:27Gregory Piazza on The Role of D-Dimer in VTE Evaluation
-
Jan 9, 2026, 07:19Emmanuel J Favaloro Shares the 1st Article in a VWD Series Marking the 100-Year Anniversary
-
Jan 9, 2026, 07:07Taylor Robichaux on Recombinant ADAMTS13 Therapy in cTTP
-
Jan 9, 2026, 06:25Sara Ng: VTE Continues to Plague Modern Oncology
-
Jan 9, 2026, 06:12James Caldwell on The Hidden Culprit in Perimesencephalic SAH
