
Wolfgang Miesbach on AAV5 Antibodies in Hemophilia B Gene Therapy
Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital, shared a post on LinkedIn:
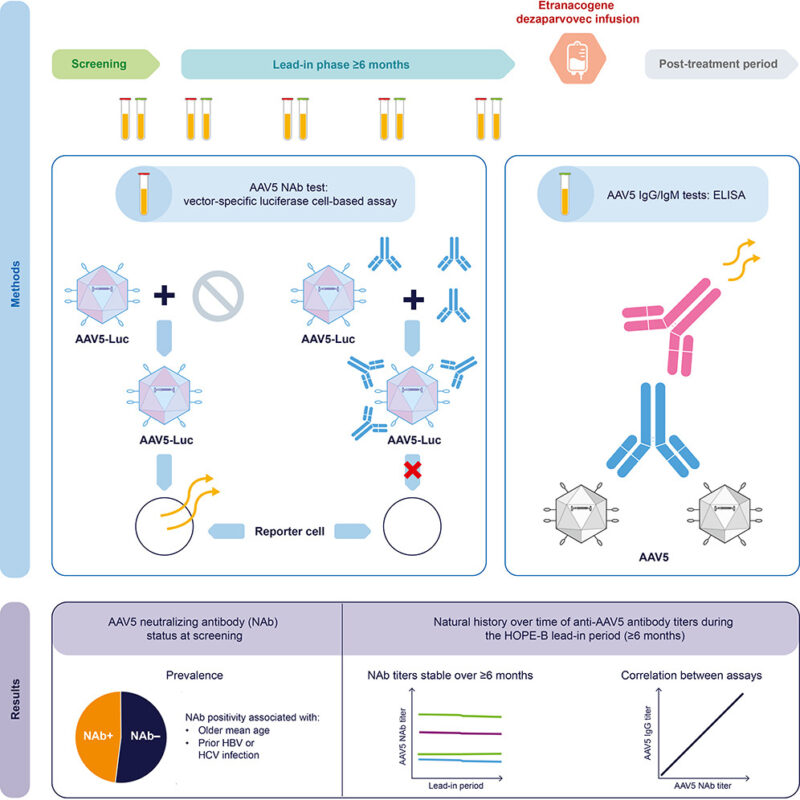
“New study on AAV5 antibodies in haemophilia B gene therapy. We just published in Molecular Therapy – Methods and Clinical Development on how AAV5 antibodies behave over time in adults with haemophilia B.
This analysis is based on the HOPE-B phase 3 trial with 67 patients in the US and Europe.
Importantly, for etranocogene dezaparvovec, AAV5 NAb positivity is not a strict contraindication. But very high titers may reduce transduction efficiency. That’s why NAb screening is an essential part of assessing eligibility for commercial gene therapy.
Key findings:
- 48% of patients had neutralizing antibodies (NAbs) at screening (median titer 58; range 9–3440)
- Levels remained stable over 8 months ( median variation 25%; range 2–154%)
- Strong correlation between NAbs and IgG (r=0.96)
- Fewer than 5% switched from negative to positive
- ~10% seroreverted from positive to negative
- NAb positivity was more frequent in patients ≥50 years (p=0.0065)
Why this matters?
NAb test results were consistent over months. Screening can be done up to 8 months before gene therapy infusion, giving more flexibility for clinical planning.
Thanks to Robert Klamroth and Sandra Le Quellec for leading this work, and to CSL for supporting the study.”
Read the full article here.
Article: Natural history of preexisting AAV5 antibodies in adults with hemophilia B during the lead-in of the etranacogene dezaparvovec phase 3 study
Authors: Robert Klamroth, Michael Recht, Nigel S. Key, Wolfgang Miesbach, Steven W. Pipe, Radoslaw Kaczmarek, Douglass Drelich, Blanca Salazar, Sandra Le Quellec, Paul E. Monahan, Nicholas Galante, Paul van der Valk, Jacqueline Tarrant
Stay updated on all scientific advances in the field of Hemophilia with Hemostasis Today.
-
Feb 23, 2026, 18:13Fight4Hematology Supports Research and Empowers the Next Generation – ASH
-
Feb 23, 2026, 17:59Wolfgang Miesbach: Real-World Evidence of Emicizumab on Joint Outcomes in Hemophilia A
-
Feb 23, 2026, 17:56Shiny K Kajal: The Transfusion Reaction We Often Miss
-
Feb 23, 2026, 17:53Radheshyam Meher: Contributing to the Transfusion Evidence Round-Up for International Childhood Cancer Day 2026
-
Feb 23, 2026, 17:46Mahesan Subramaniam: The Physiological Impact of Anger on Immunity
-
Feb 23, 2026, 17:42Bryan Fry: First Evidence That Bothrops atrox Venom Directly Activates Human Factor VII
-
Feb 23, 2026, 17:34Bastu Odoka: Why Blood Should NOT be Left at the Bedside to ‘Warm’
-
Feb 23, 2026, 17:28Henry Burkitt: Patients Are Challenging How the Medicines Policy System Works in England
-
Feb 23, 2026, 16:50Mutaz Al‑Sabah: Interesting Webinar on FH in Women is Now Available to Watch

