
David McIntosh: Making Measurable Progress – Towards a World in Which No-one Ever Dies from Lack of a Vital Plasma-Derived Medicine
David McIntosh, Founder and Chair of United Plasma Action, shares Reflections on International Plasma Awareness Week – in Week one of the “Next Year” with Hemostasis Today:
”As the justifiable excitement of International Plasma Awareness Week fades away and the international plasma medicines community settles down to the hard global slog of delivering on the key messages, it seems appropriate to stand back for a moment and reflect on last week and the implications for patients, globally.
International Plasma Awareness Week has become an annual showcase event.
It highlights the importance of plasma-derived medicines for the treatment of seriously ill patients.
It also draws particular attention to the vital importance of plasma donors, and the great debt we owe to these valiant volunteers for what they do.
Of course, without willing human donors to provide the essential raw material, no plasma-derived medicines would be possible.
Equally essential are the fine teams of both corporate and public service staff who make plasma collection and processing possible, in many countries in the World.
They too are deservedly celebrated during International Plasma Awareness Week, each year.
Amidst these annual celebrations there is always an underlying hope that the week’s events will help bring the benefits of plasma-derived medicines to patients for whom such treatments have not so far been made available.
What is rarely mentioned, and is very little understood, is that, globally, patients who do not have access to the plasma-derived medicines they need, outnumber the ones who do by about 4 to one.
In many parts of the World, the consequent suffering and death is heart-breaking.
To help bring the situation into perspective, it’s sobering to view the wide disparities in plasma awareness and action in different parts of the World.
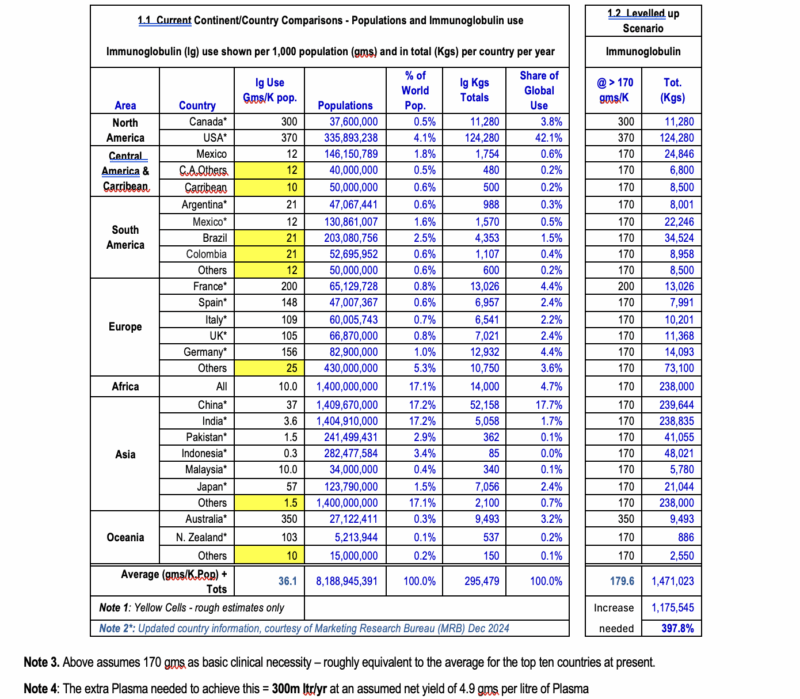
Figure 1, below, gives a brief summary of the situation, taking Immunoglobulin (Ig) as an illustrative example. (An absolutely vital treatment for patients with congenital and acquired immunodeficiencies, neurological, oncological and other conditions, Immunoglobulin, like all plasma-derived medicines, is in desperately short supply globally.)
Fig.1 is set out in two sections – showing the current situation (left) and the situation that could arise if all nations could be levelled up, to at least reach a basic Ig availability of 170 gms per thousand population (right).
The exact level to which the World should aspire, to achieve effective care and true Health Equity in this field, is of course arguable.
An exact specification is not required.
The only immediate necessity is a target of “More – Much More” – “As Soon as Possible – PLEASE!”.
The 170gm aspiration is chosen here as a reasonable guess at the minimum necessary for an appropriate level of clinical availability – based on the average figure for the top ten Ig-using countries. (As a comparator, all of the top 3 countries have over 300gms available per year, per thousand population.)
To achieve this level of reasonable clinical availability of essential plasma-derived medicines, worldwide, will require an increase in the annual global plasma “harvest” of some 300 million litres (at current levels of process yield).
That may sound like a lot.
Indeed, it would (will..) quadruple the current global total.
That’s what’s required if the present-day suffering and death is to be alleviated.
In perspective though, 300m litres equates to a “street value”, globally, of some $60 bn US – not such a huge amount in geopolitical terms.
In the life/death equation, for many millions of patients, $60bn would surely be VERY good value for money!
So, the follow-on challenge in the year ahead is to chart a course to Plasma Awareness Week 2026 that comes alive in a different World altogether – a World in which awareness of plasma, plasma-derived medicines and the crying need for more of them, is even more widely understood, and is being much more widely acted on.
The end point must surely be a World in which all nations have committed themselves to ensuring national self-sufficiency in the availability of pharmaceutical grade plasma, have invested in that goal and are making measurable progress – towards a World in which no-one ever dies from lack of a vital plasma-derived medicine.”
More on the topic featured in Hemostasis Today.
-
Feb 1, 2026, 13:32Shiny K. Kajal: A Series On Reaching the Diagnosis of Immune Thrombocytopenia
-
Feb 1, 2026, 13:26Mary Cushman: Association of Atrial Fibrillation with Risk of Incident Cognitive Impairment
-
Feb 1, 2026, 13:23Abdulrahman Katib: Extended Low-Dose DOAC May Reduce VTE Recurrence
-
Feb 1, 2026, 13:22Jeff June: The Stroke That Wasn’t New – Why ‘Cryptogenic’ Often Means Undetected
-
Feb 1, 2026, 13:11Panagiotis Doukas: Plasma Desmosine as a Biomarker of Increased Proteolytic Activity in the Aortic Wall
-
Feb 1, 2026, 12:54Pierpaolo Di Micco: Starting a New Era for Anticoagulant Treatments
-
Feb 1, 2026, 12:16Erwin Loh: Immunotherapy Reduces Plaque in Arteries of Mice
-
Feb 1, 2026, 12:06Ney Carter Borges: The Central Role of Endothelial Dysfunction in Cardiovascular Disease Progression
-
Feb 1, 2026, 12:05Causes of Anemia – A Quick Overview From Mahnoor Shah

