
Wolfgang Miesbach;Robert Klamroth/LinkedIn
Feb 13, 2026, 11:00
Wolfgang Miesbach: 5 Year Liver Safety Outcomes After Gene Therapy for Severe Haemophilia A
Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital, shared on LinkedIn:
“Impressed by the detailed safety focus in EAHAD2026 session on valoctocogene roxaparvovec gene therapy for severe haemophilia A.
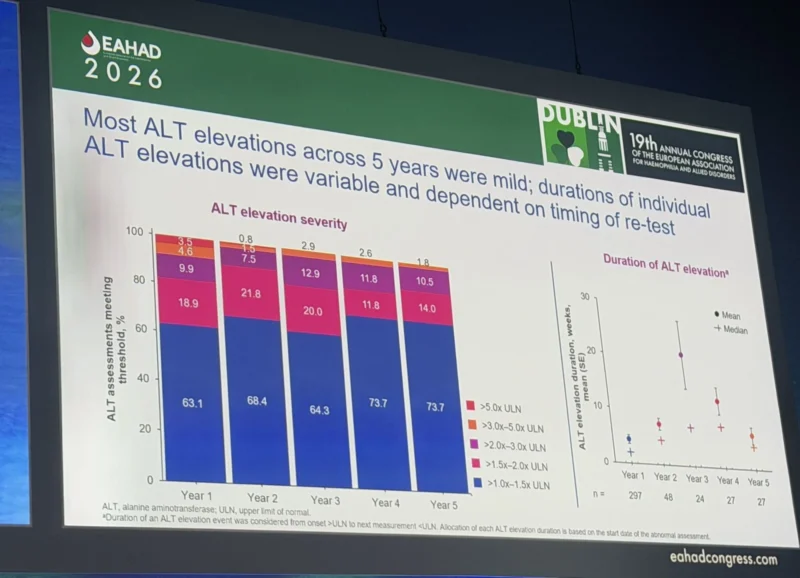
Robert Klamroth presented a 5‑year characterisation of ALT elevations in the phase 3 GENEr8‑1 trial, offering very concrete insights into liver safety after gene transfer:
- ALT elevations were the most common adverse event, occurring in most participants in year 1 after infusion, then falling sharply and stabilising in years 2–5.
- The vast majority of ALT elevations were mild (≤3× ULN), asymptomatic and transient, with events >5× ULN being rare and no clear signal for progressive liver injury.
- Glucocorticoids were used predominantly in years 1–2 and only in those with ALT >ULN, allowing targeted management of liver enzyme rises while avoiding prolonged steroid exposure for most patients.
- Over the full 5‑year period, the safety profile remained consistent with earlier read‑outs, supporting durable bleed protection and quality‑of‑life gains with valoctocogene roxaparvovec as a one‑time treatment option.
These data reinforce that careful monitoring, clear steroid algorithms and long‑term follow‑up are essential to delivering AAV gene therapy.”
Find more posts featuring Wolfgang Miesbach on Hemostasis Today.
-
Feb 13, 2026, 13:59Jeff June: Why Women’s Health Requires a New Stroke Intelligence Layer
-
Feb 13, 2026, 13:57Amy Rivera: Understanding Lymphedema Imaging
-
Feb 13, 2026, 13:53What if Hemophilic Joint Damage Isn’t as Irreversible as We Thought? – RPTH Journal
-
Feb 13, 2026, 13:52Wolfgang Miesbach: Adding Years of Life – and Life to Years
-
Feb 13, 2026, 13:14Eirini Kontou: Launch of the e-Optimism Research Study to Support Life After TIA and Minor Stroke
-
Feb 13, 2026, 13:11Omar Adwan: Understanding ITP, TTP, and DIC
-
Feb 13, 2026, 13:01Abhishek Kumar: The Future of Uncontrolled Bleeding Therapies and the Evolving Hemostasis Pipeline
-
Feb 13, 2026, 12:49Simon Senanu: ‘Within Range’ Is Not the Same as ‘Safe’ in Laboratory Medicine
-
Feb 13, 2026, 12:35Heghine Khachatryan: Advances in Targeted Therapy Transforming TTP Management at ASH Highlights 2026

