
Vikas Londhe on Andexxa’s Safety Assessment Updates
Vikas Londhe, Chief Editor of Pharmacally.com, shared on LinkedIn:
”FDA Safety Update: Andexxa Risks Outweigh Benefits After Postmarketing Thromboembolic Events—Market Withdrawal Planned
FDA Updates Safety Assessment of Andexxa After Serious Thromboembolic Events.
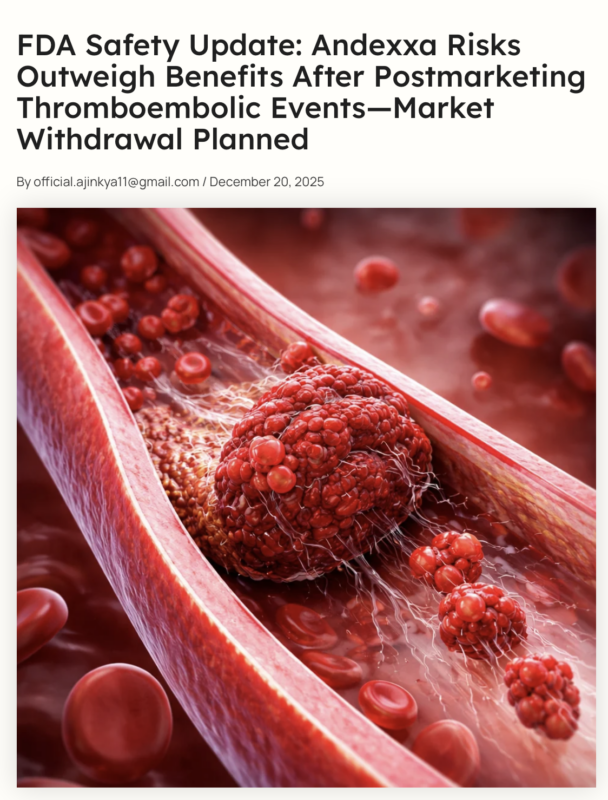
On December 18, 2025, the U.S. Food and Drug Administration (FDA) issued a safety communication regarding Andexxa (coagulation factor Xa (recombinant), inactivated-zhzo).
The agency has reviewed postmarketing safety data showing an increased incidence of thromboembolic events, including serious and fatal outcomes, in patients treated with Andexxa.
Based on these findings, the FDA now considers that the risks associated with Andexxa outweigh its benefits in its approved use.”
Read the full announcement here.
Stay updated with Hemostasis Today.
-
Feb 17, 2026, 09:04Mahesan Subramaniam: A Heart Attack Is a Long Story That Ends Fast
-
Feb 16, 2026, 15:44Lakmali Silva: Silva Lab Showcases Research at the Gordon Research Conference on Plasminogen and Extracellular Proteolysis
-
Feb 16, 2026, 15:36Heghine Khachatryan: Strengthening Maternal Resilience Against Postpartum Hemorrhage
-
Feb 16, 2026, 15:34Sevak Mirabyan: Advancing Cancer Care Through Knowledge, Collaboration, and Early Diagnosis in Armenia
-
Feb 16, 2026, 15:29Chokri Ben Lamine: Key Clinical Insights of Drug Induced Thrombocytopenia
-
Feb 16, 2026, 15:28Ney Carter Borges: Coronary Intravascular Lithotripsy – Clinical Performance and Safety
-
Feb 16, 2026, 15:24Flora Peyvandi: Highlighting Overdose, Homicide, and Suicide as Causes of Maternal Death in the US
-
Feb 16, 2026, 14:53Wafaa Abougabal: Differential Diagnosis of Acute Neurologic Deficits and Stroke in PICU
-
Feb 16, 2026, 14:49Nayab Ahmed: Synthesis of ATP and Its Significance in Storage of Blood

