
Historic FDA Win: Pembrolizumab + EV Becomes New Standard for Cisplatin-Ineligible MIBC
Rishabh Jain, Medical Oncologist at AIIMS Delhi, posted on X:
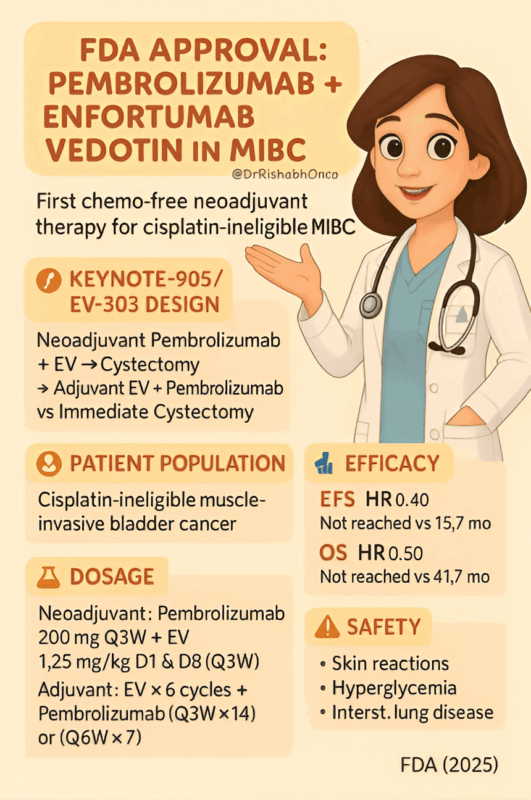
”FDA APPROVAL – MIBC
Pembrolizumab + Enfortumab Vedotin gets approved as neoadjuvant → adjuvant therapy for cisplatin-ineligible muscle-invasive bladder cancer (MIBC)
Why this matters?
For the first time ever, a chemo-free neoadjuvant regimen improves BOTH EFS and OS in MIBC patients who cannot take cisplatin.
This is a new standard for a previously undertreated group.
KEYNOTE-905 / EV-303
Design:
Neoadjuvant Pembrolizumab + EV → cystectomy → adjuvant EV + Pembrolizumab → Pembrolizumab alone
vs
Immediate cystectomy alone
Population: Cisplatin-ineligible or cisplatin-declining MIBC patients.
Efficacy Highlights
EFS:
• Not reached vs 15.7 months
• HR 0.40 (60% risk reduction)
OS:
• Not reached vs 41.7 months
• HR 0.50 (50% risk reduction)
These are huge survival deltas for localized bladder cancer.
Regimen
Neoadjuvant (9 weeks):
• Pembrolizumab 200 mg Q3W
• EV 1.25 mg/kg D1 & D8 (Q3W) × 3 cycles
Adjuvant:
• EV × 6 cycles + Pembrolizumab (Q3W ×14 or Q6W ×7)
• Then Pembrolizumab alone → Total adjuvant duration 42 weeks
Safety Snapshot
Similar to prior EV + Pembro experience:
• Skin reactions
• Hyperglycemia
• ILD/pneumonitis
• Peripheral neuropathy
• Immune-related AEs from pembrolizumab
Takeaway
A practice-changing, chemo-free perioperative IO-ADC strategy for MIBC.
This is likely to shift guidelines fast – especially for cisplatin-ineligible patients.
Source: FDA (2025)”
Stay updated with Hemostasis Today.
-
Jan 26, 2026, 05:40Vicki Kopplin Offers Immediate Funding for People With Bleeding Disorders
-
Jan 26, 2026, 05:27Jill Storry on Alloimmune Hemolytic Disease of the Fetus And Newborn
-
Jan 26, 2026, 05:09Wathsala Manindrani Gives a A Practical Perspective on Red Cell Exchange Transfusion
-
Jan 26, 2026, 04:59Abdul Mannan: BDUC – 4 Letters That Make Many Haematologists Uncomfortable
-
Jan 26, 2026, 04:51Manoj Kumar Singh: Power In Me Foundation Celebrates 2026 as Year Of Rare
-
Jan 26, 2026, 04:40Heghine Khachatryan: Did You Know VWD Comprises 3 Main Types?
-
Jan 25, 2026, 15:57Céline Chapelle Shares Clinical Predictors From the API-CAT Trial
-
Jan 25, 2026, 15:42Francesco Lo Monaco on Heart Disease Starting Quiet While Your Labs Speak First
-
Jan 25, 2026, 15:25Muhammad Ibrahim on Efficacy and Safety of Extended DOACs Use in VTE
