
Wolfgang Miesbach Shares Newly Released Gene Therapy Qualification Criteria Paper
Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital, shared a post on X:
“Exciting News! Our opinion paper on gene therapy qualification criteria has just been published! It outlines comprehensive standards for hub-and-spoke centers delivering gene therapy for haemophilia, helping ensure safety and efficacy across Europe and beyond:
Timely infrastructure guidance: The paper establishes practical qualification criteria for haemophilia centres delivering gene therapy, helping address new organizational, technical, and clinical requirements as gene therapies move into real-world practice.
Expert consensus: A comprehensive survey achieved 100% response among interdisciplinary specialists, ensuring robust recommendations grounded in broad clinical experience.
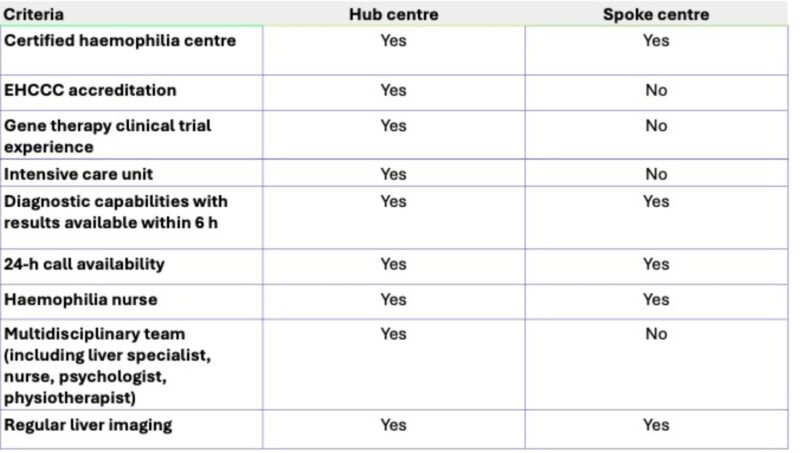
Hub-and-spoke model: The publication details a scalable and flexible framework—hub centres manage dosing and complexity while spoke centres provide local follow-up—making advanced therapies feasible and accessible across diverse care settings.
Unprecedented multidisciplinary team requirements including gene therapy liaison nurses, specialized pharmacists, administrative coordinators, and mandatory access to hepatologists.
Clear operational standards: The authors provide actionable criteria (e.g., bioisolator for ATMPs, 24-hour on-call service, rapid laboratory turnaround) and delineate staff roles, allowing centres to objectively assess readiness.
Flexible and adaptive: The model accommodates regional differences and supports spoke centre capacity-building, allowing responsibility to shift as local experience increases—a practical approach for evolving health systems.
Focus on safety and coordination: Emphasizes standardized protocols, scenario preparedness (“dry run” exercises), and robust SOPs for adverse event management, underpinning a safety-first transition to gene therapy.
Globally relevant: While based on European experience, the findings and framework are adaptable to international settings, with comparisons to other models such as the US health system.
Congratulations and a BIG THANK YOU to the EAHAD Gene Therapy Working Group!”
For more information follow the link.
Stay informed with Hemostasis Today.
-
Jan 22, 2026, 15:36We Must Roll Up Our Sleeves And Help: José Antonio García Erce on Plasma Donation
-
Jan 22, 2026, 15:25Nita Radhakrishnan on Challenges In Congenital Afibrinogenemia
-
Jan 22, 2026, 15:10Jin Q Gives a Summary of 2025’s Most Impactful Cell and Gene Therapy Milestones
-
Jan 22, 2026, 14:57Nirav Dhanesha on CD14 Acting As A Functional Driver of DVT
-
Jan 22, 2026, 11:41Jamilla Goedegebuur and Colleagues on VTE Management in Case of PAD
-
Jan 22, 2026, 11:28Abdulrahman Katib on API-CAT Trial’s Evaluation of Apixaban Dosing
-
Jan 22, 2026, 11:19Bruno Odisio: Ablation Margins Are Software-Dependent
-
Jan 22, 2026, 10:38Pedro Perez: The VTE Market Is Clearly Entering Its Next Phase
-
Jan 22, 2026, 10:29Marvin Garcia Reyes Presents a Case of Aorto-Visceral and Aorto-Iliac Thrombosis
