
Amol Akhade: ALASCCA Trial – Aspirin as Precision Adjuvant in Colorectal Cancer?
Amol Akhade, Senior Medical and Hemato-Oncologist at Fortis Hospital Mumbai and Founder of Suyog Cancer Clinics, shared a post on LinkedIn:
”NEJM 2025: ALASCCA Trial – Aspirin as Precision Adjuvant in Colorectal Cancer?
The ALASCCA trial (Martling et al., NEJM 2025) is a landmark step in repurposing a cheap, globally accessible drug — aspirin — within the framework of precision oncology.
Trial design:
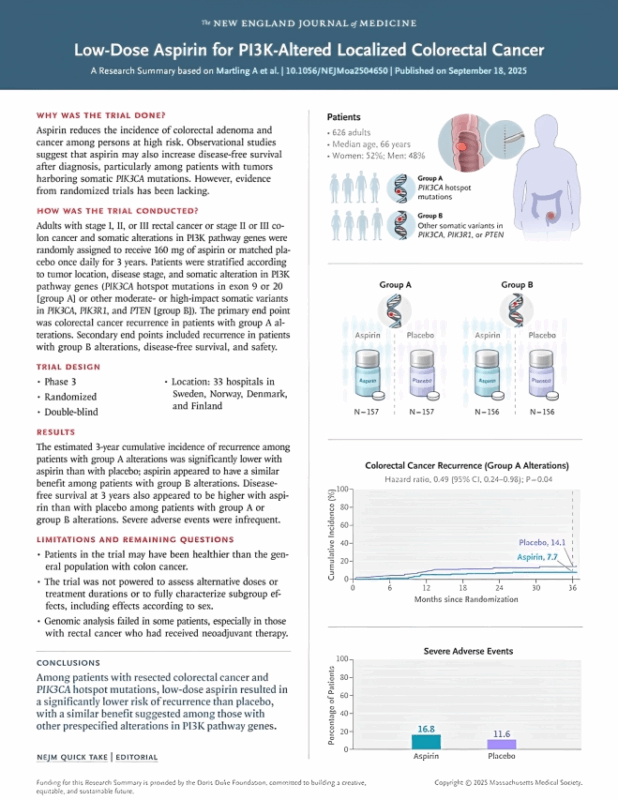
- 6,397 patients screened → 2,980 sequenced → 1,103 (37%) harbored PI3K-pathway alterations. Hotspot PIK3CA exon 9/20 mutations: 17% Other PI3K/PTEN/PIK3R1 variants: 20%
- 626 randomized to aspirin 160 mg vs placebo for 3 years, double-blind, registry-based.
Results:
- In hotspot (Group A): recurrence 7.7% vs 14.1% (HR 0.49, p=0.04). In other PI3K variants (Group B): recurrence 7.7% vs 16.8% (HR 0.42).
- Disease-free survival trended positive, but overall survival benefit not shown yet
Number Needed to Treat (NNT):
- Colon stage II → weak (NNT 42, ARD ~2%). Colon stage III → strong (NNT 9, ARD ~12%). Rectum stage III → best (NNT 6, ARD ~17–20%). Stage I/II rectum → modest, inconsistent benefit.
Safety:
- Severe AEs: 16.8% with aspirin vs 11.6% with placebo. Most common: GI bleeding, thromboembolism, post-op complications. One aspirin-related death reported.
Critical view:
- Strengths: biomarker-driven design, pragmatic registry-based RCT, global relevance of a low-cost intervention.
Limitations: borderline statistical significance (p=0.04 in Group A), wide confidence intervals, subgroup heterogeneity (sometimes non-hotspot mutations outperformed hotspots), and no OS benefit yet.
Clinical implication: Most compelling signal: stage III rectal & colon cancers. Stage II benefit marginal. Aspirin may join the armamentarium in high-risk, biomarker-selected subgroups, but universal use is premature.
Editorial take (Goldberg and Meyerhardt, NEJM):
They argue that biomarker-guided aspirin could mark long-awaited progress in adjuvant CRC therapy, similar to MSI-high immunotherapy (ATOMIC trial). But careful balancing of recurrence reduction vs bleeding risk is essential.
Bottom line:
- The ALASCCA trial demonstrates the promise of repurposed, low-cost drugs guided by genomics. Aspirin halves recurrence in PI3K-altered CRC, but the benefit is heterogeneous and not yet practice-changing.
Future pooled analyses and replication will decide if aspirin truly becomes a standard precision adjuvant.”
Stay updated with Hemostasis Today.
-
Feb 23, 2026, 12:02Marios Georgakis: An Unprecedented for An Antithrombotic Therapy from OCEANIC-STROKE Trial
-
Feb 23, 2026, 11:37Charles Okyere Boadu: Blood Donation Helps Lower Your Risk of Stroke and Organ Damage
-
Feb 23, 2026, 11:29Emma Lefrancais: Uncovering A Key Role for The IL-33/ST2 Axis in Platelet Biology with Lucie Gelon
-
Feb 22, 2026, 14:16Ilenia Calcaterra: From Representation to Intellectual Independence in Women in Science
-
Feb 22, 2026, 13:27Pete Stibbs: New AHA and ACC PE Guidelines Finally Align with Real Clinical Practice
-
Feb 22, 2026, 10:39Tagreed Alkaltham: Fibrinogen Concentrate Is a Deliberate Clinical Choice in Acute Bleeding
-
Feb 22, 2026, 09:38Abdulrahman Nasiri: Significant Shifts In The 2026 AHA/ACC Guidelines for Acute Pulmonary Embolism
-
Feb 22, 2026, 09:22Shiny K. Kajal: Not All Transfusion Reactions Are Immunohematologic Incompatibilities
-
Feb 22, 2026, 09:12Arun V J։ The Hidden Risks in Every Blood Bag

