
Nicolas Gendron on The Emerging Role of CLIA in The Diagnosis Strategy for HIT
Nicolas Gendron, Research Fellow, Hematologist at Boston Children’s Hospital, shared a proud post on LinkedIn:
”New online publication in Blood VTH, Blood Journals Portfolio
Heparin-induced thrombocytopenia (HIT) is a serious complication of heparin treatments.
Its rapid and reliable diagnosis is essential to adapt management and improve the prognosis of patients.
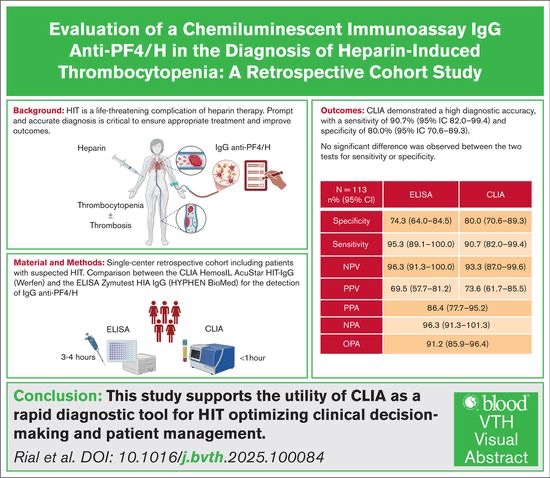
At the HEGP, we compared the diagnostic performance for the detection of anti-PF4/H antibodies of the HemosIL AcuStar HIT-IgG test (CLIA, Werfen ) with that of our Zymutest HIA IgG reference test (ELISA, HYPHEN BioMed ), in 113 patients suspected of TIH (score 4Ts >3), taking as a reference the result of the platelet function test by radiolabeled serotonin release (RAS).
Excellent sensitivity and specificity of both tests
- Very few discordant cases among patients with confirmed HIT
- Overall agreement between the two techniques: 91.2%
- CLIA has proven to be reliable, fast and efficient
These findings support the use of CLIA as a rapid and relevant diagnostic tool in the diagnosis strategy for HIT
Congratulations to Carla Rial and everyone involved in this great collaborative work!
Shaya Sable, Christine Le Beller, Aurélie Sarthou, Mauge Laetitia, Justine Piazza, Roya Nili Maude Laney, Isabelle Presot Jean-Luc Diehl, Agnès Lillo-Le Louet, Anne Godier, David Smadja, Helley-Russick Dominique
Paris Cardiovascular Research Center – PARCC
Université Paris Cité Faculté de Santé – Université Paris Cité
Université Paris Cité Médecine Greater Paris University Hospitals – AP-HP
Werfen Werfen North America”
Read the full article in Blood VTH.
Article: Evaluation of a chemiluminescent immunoassay IgG anti-PF4/H in the Diagnosis of Heparin-Induced Thrombocytopenia
Authors: Carla Rial, Nicolas Gendron, Christine Le Beller, Shaya Sable, Aurélie Sarthou, Laetitia Mauge, Justine Piazza, Roya Nili, Maude Laney, Isabelle Presot, Jean-Luc Diehl, Anne Godier, Agnès Lillo-Le Louet, David M. Smadja, Dominique Helley
Stay updated on the latest scientific advances in the field of platelet disorders with Hemostasis Today.
-
Jan 30, 2026, 11:38Aaron Rodriguez Calienes on Intracranial Stenting: Rescue vs First-Line Outcomes
-
Jan 30, 2026, 11:25Ahmed Nasreldein on Sex Disparities in Thrombolysis Delay Among Egyptian Stroke Patients
-
Jan 30, 2026, 11:17Yanki Yarman: My PhD Project Has Been Published in Blood!
-
Jan 30, 2026, 11:08Alexandros Apostolou on Complications Associated with IDU and Thrombophilia
-
Jan 30, 2026, 10:57Peisong Ma on GRK5 Polymorphism Affecting Platelets
-
Jan 30, 2026, 10:47Ashok Yadav Explains Fetal Thrombotic Vasculopathy: FTV
-
Jan 30, 2026, 10:34Saif ur Rahman Compares K2 and K3 EDTA: Key Differences Explained
-
Jan 30, 2026, 10:22Manpreet Gill on Heparin Resistance: When 14 Years of Experience Finally Gets a Plot Twist
-
Jan 30, 2026, 10:14Mohamed Elsaid on The Role of Hyperlipidemia in Thrombogenesis

