C-Terminal VWF Variants in von Willebrand Disease: Functional Insights from the D4-C6 Domains
The RPTH Journal (Research and Practice in Thrombosis and Haemostasis) shared an insightful post on LinkedIn:
”A small panel of VWF variants were investigated for expression and function – and how they might cause Von Willebrand Disease.
Golzar Mobayen and team reveal that the D4 and C-domains of VWF might play a role!
Other Authors: Sammy El-Mansi, Alain Chion, Tom Nightingale, and Tom McKinnon.”
Read the full article here.
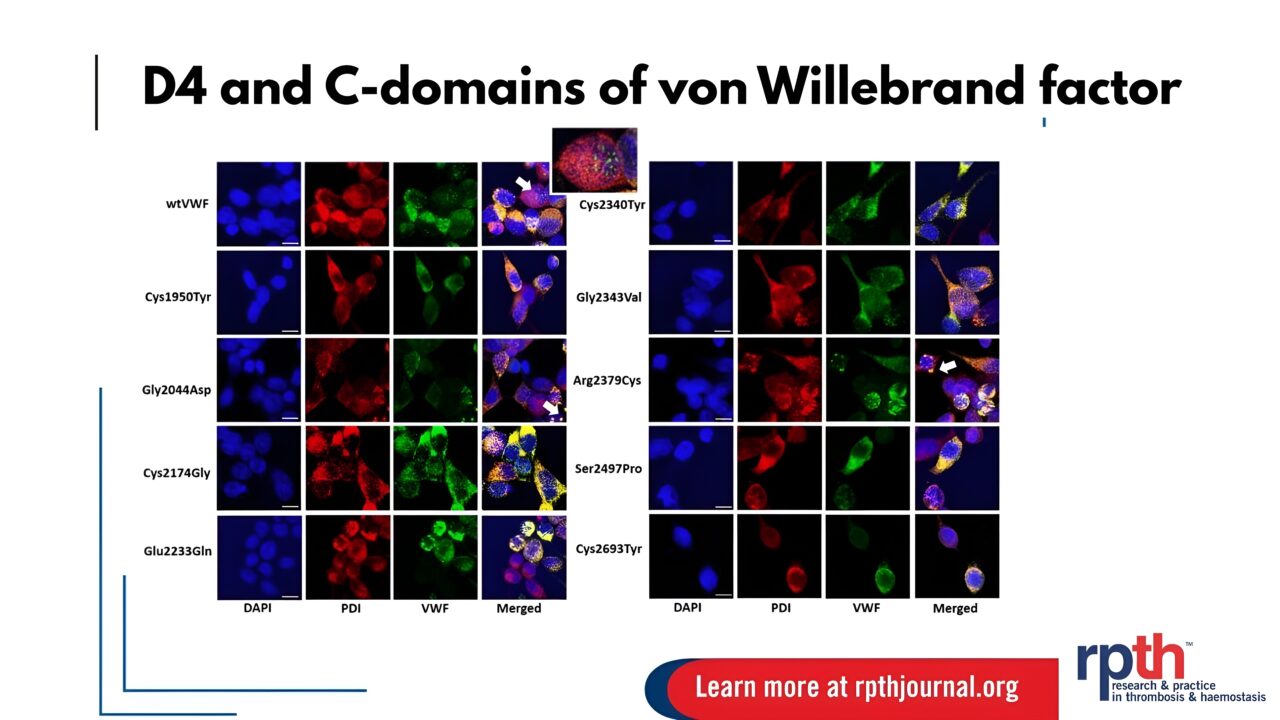
The ”Probing rare von Willebrand disease–causing mutations in the D4 and C-domains of von Willebrand factor” study published by Golzar Mobayen et al. in the RPTH Journal, sheds light on the possible roles of vWF C-terminal D4-C6 domains in development of VWD.
Von Willebrand disease (VWD) arises from quantitative or qualitative deficiencies in von Willebrand factor (VWF), with a broad spectrum of genetic variants contributing to disease pathology.
However, mutations within the C-terminal D4-C6 domains of VWF remain under-characterized.
The findings demonstrate that variants in this region can significantly impair VWF expression and function.
Furthermore, the phenotypic impact varies depending on zygosity, with distinct differences observed between homozygous and heterozygous states.
These insights underscore the importance of the VWF C-terminal domains in maintaining normal hemostatic function.
All the latest scientific advancements on bleeding disorders featured in Hemostasis Today.
-
Jan 27, 2026, 13:48Filippo Cademartiri: 3 Biomarkers Are Better Than 1: Refining ASCVD Risk in MESA
-
Jan 27, 2026, 12:27Martin Haluzík on Residual Cardiovascular Risk in Coronary Artery Disease
-
Jan 27, 2026, 12:18Stuart Phillips: Strong Bodies Are Good, Informed Bodies Are Better!
-
Jan 27, 2026, 12:07Daniel Torrent: This Should Be An Ice Cold Take… But Veins Aren’t Arteries
-
Jan 27, 2026, 09:17Paolo Zamboni on CCSVI Associated to Multiple Sclerosis
-
Jan 27, 2026, 09:02Rowan Paul: Platelet Dose Determines Success in PRP Therapy
-
Jan 27, 2026, 08:48Uriel Suárez: Hemi‐Orolingual Angioedema in a Patient With VEXAS Syndrome
-
Jan 27, 2026, 08:23Rossella Crescitelli on Platelets’ and PEVs’ Association with Lung Cancer Metastases
-
Jan 27, 2026, 08:11Matthew Flick Links Urokinase Plasminogen Activator Deficiency to Obesity
