
Khaled Musallam Shares BEYOND Trial Phase 2 Insights on Luspatercept Use in NTD β-Thalassaemia
Khaled Musallam, Group Chief Research Officer at Burjeel Holdings, shared on LinkedIn:
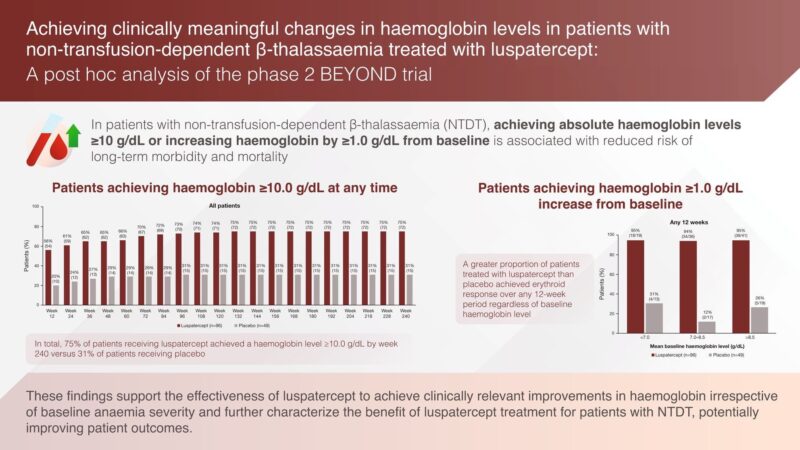
”Pleased to share our new paper out today in the British Journal of Haematology.
This is a post-hoc analysis of the BEYOND trial specifically looking at the proportion of NTDT patients that reached and sustained a hemoglobin level of >10 g/dL while on ß, reflecting clinically meaningful impact considering this would now place them in the ‘safe zone’.”
Read the full article in BJHaem.
Article: Achieving clinically meaningful changes in haemoglobin levels in patients with non-transfusion-dependent β-thalassaemia treated with luspatercept: A post hoc analysis of the phase 2 BEYOND trial
Authors: Khaled Musallam X, Ali T. Taher, John B. Porter, Antonis Kattamis, Mrudula B. Glassberg, Luciana Moro Bueno, Patricia Martin-Regueira, Marta Reverte, Loyse Felber Medlin, Matthew Dyer, Kefeng Wang, Maria Domenica Cappellini
Stay updated on all scientific advances with Hemostasis Today.
-
Jan 29, 2026, 05:22Aravind Palraj Draws a Comparison Between APS and Lupus Cerebritis
-
Jan 29, 2026, 05:09Edina Cenko on Expanding Cardiovascular Risk: From Ischaemia to Emerging Frontiers
-
Jan 29, 2026, 04:58Anna Randi Shares the Latest Episode of The VWF and Angiogenesis Story from Her Lab
-
Jan 29, 2026, 04:49Ruah Alyamany on Her Contribution to Establishing The Role of IgM in APS
-
Jan 29, 2026, 04:42Jecko Thachil on ‘Break Through’ Thrombosis Despite DOAC Use
-
Jan 28, 2026, 13:55Ney Carter Borges on Colchicine As 2ry Prevention in Atherosclerotic CVD
-
Jan 28, 2026, 13:35Louise St Germain Bannon Invites You to Submit Your Work to JTH and RPTH
-
Jan 28, 2026, 13:26Livia Stanger and Colleagues Link CS014 to in Vivo Thrombosis
-
Jan 28, 2026, 13:14Robert Campbell Congratulates Izabella Andrianova for Her Recent Publication in JTH
