
Abdul Mannan – Breaking in Haem: Andexanet Alfa Withdrawn from US Market
Abdul Mannan, Consultant Haematologist at Betsi Cadwaladr University Health Board, shared on LinkedIn:
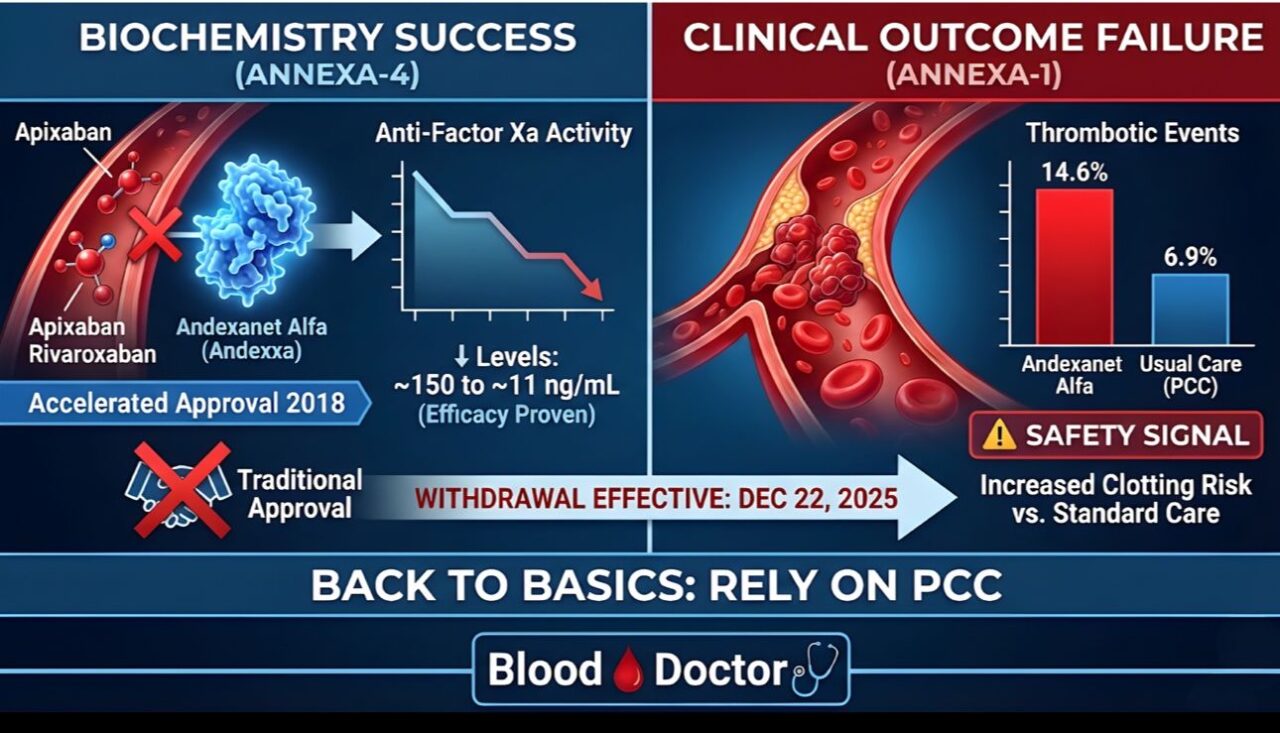
“Breaking in Haem: Andexanet Alfa Withdrawn from US Market
A major shift in anticoagulation reversal is happening. AstraZeneca is withdrawing the BLA for Andexxa (andexanet alfa), effective Dec 22, 2025.
Why is this happening? Let us make it simple
It’s a case of “Biochemistry vs. Clinical Outcome.”
We failed to bridge the gap from Accelerated Approval (2018) to Traditional Approval.
The Data Dilemma:
Efficacy: It worked. In ANNEXA-4, it slashed anti-Factor Xa activity (e.g., Apixaban levels dropped from ~150 to ~11 ng/mL).
Safety: The ANNEXA-I trial showed a worrying signal. Thrombotic events were 14.6% with Andexanet vs 6.9% with usual care (PCC).
What now?
We return to the basics. For life-threatening bleeding on apixaban or rivaroxaban, we rely on Prothrombin Complex Concentrates (PCC).
A reminder that even ‘targeted’ antidotes must prove they don’t cause more harm than the bleeding they are trying to stop.”
More posts featuring Abdul Mannan on Hemostasis Today.
-
Feb 6, 2026, 19:05Shinya Goto: Another Important ABC Risk Score for AF Paper
-
Feb 6, 2026, 18:52Pascal Mensah: Why Inflammation Loves Redox Entropy?
-
Feb 6, 2026, 18:49Additional Oxerutin Therapy to Promote Deep Vein Thrombus Resolution – JTH
-
Feb 6, 2026, 18:47Srishti Goyal: Blood Donation – The Health Benefits No One Talks About
-
Feb 6, 2026, 18:42Jim Hoffman: Targeting Extracorporeal Removal of Extracellular Chromatin to Mitigate Lupus Pathogenesis
-
Feb 6, 2026, 18:37Erwin Loh: High-Salt Diet Linked to Inflammation and Memory Loss by Altering the Microbiome
-
Feb 6, 2026, 18:31Tareq Abadl: Massive Transfusion Protocols – Speed, Balance, and Survival
-
Feb 6, 2026, 18:27Sarah-Jane Nkrumah: Giving a Wider Understanding to Those Living With Sickle Cell
-
Feb 6, 2026, 18:07Anna Kouraba: Laiko Hospital’s Contribution to rFVIII Single Chain Real-World Evidence at EAHAD 2026

