
Davide Matino at BIC 2025: Phase 3 Data on Fitelparvovec for Hemophilia A
Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital, shared a post on LinkedIn:
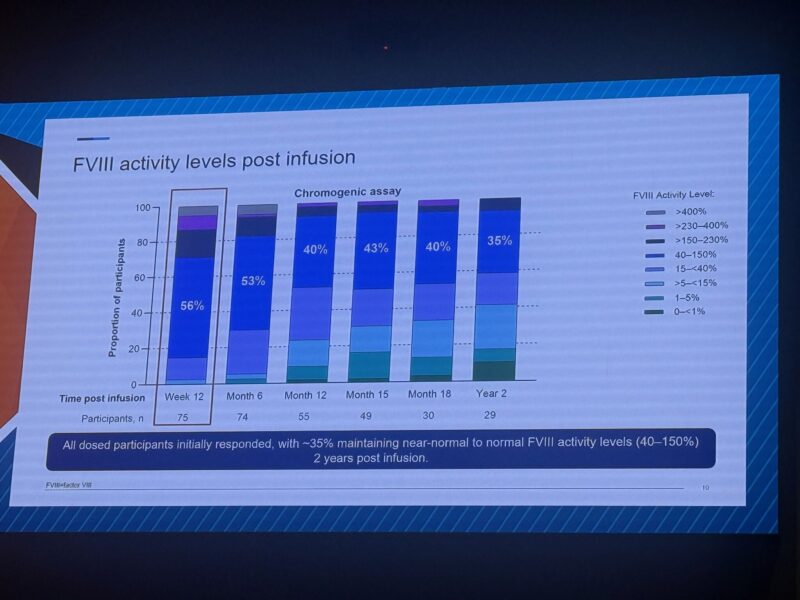
”Updated Ph3 data on gene therapy for haemophilia A with giroctocogene fitelparvovec (AAV6, 3e13 vg/kg in 74 participants), presented by Davide Matino at BIC meeting 2025:
~35% of participants maintained near-normal to normal FVIII activity (40–150%) at 2 years post-infusion
Durability data is impressive: sustained efficacy approaches three years of follow-up, demonstrating the transformative potential of this AAV6 approach
- Slow decline pattern:
– Year 1 mean: 51.7%
– Year 2 mean: 51.9% (stabilization)
– Year 3 mean: 40.7% (median: 38.0%)
Note: Only 8 participants had 3-year follow-up
- Sustained FVIII levels >5% in >80% of participants at 15 and at 24 months
- 98.3% reduction in treated bleeding episodes (4.08 → 0.07 ABR post-infusion)
- Transient FVIII activity >150% in nearly 50%, managed with low-dose prophylactic DOAC in 30%
- One participant developed a thrombotic event (history of thrombosis and risk factors)
- 2 participants resumed FVIII prophylaxis
Generally well-tolerated safety profile with manageable ALT elevations (ALT ↑ in 61%)
High rate of infusion-related reactions (75%), but no discontinuation of study drug administration
One case of new cancer at 35 months (oral SCC, not related to study drug); in total 2 malignancies reported
One case of FVIII inhibitor development (more to follow tomorrow!)
Despite these results, Pfizer has discontinued this program and terminated their collaboration with Sangamo Therapeutics in December 2024”
More from BIC 2025 featured in Hemostasis Today.
-
Feb 2, 2026, 17:54Danny Gaskin: Nominations are Open for the BBTS Transfusion Practitioner Special Interest Group Award 2026
-
Feb 2, 2026, 17:44Important Webinar on Care for Patients with iTTP – ISTH
-
Feb 2, 2026, 17:21Tagreed Alkaltham: Some Risks Don’t Look Like Risks in Healthcare
-
Feb 2, 2026, 17:16Sifat Jubaira: Effect of Prolonged Tourniquet Application
-
Feb 2, 2026, 17:14Vivek Mahto: Understanding Deep Vein Thrombosis – Causes, Symptoms, and Prevention
-
Feb 2, 2026, 17:08Tareq Abadl: Heparin vs Warfarin
-
Feb 2, 2026, 17:07Mary Cushman: New Research on Aspirin Use in Pregnancy and Stroke Risk in Offspring
-
Feb 2, 2026, 16:52Aravind Palraj: Young Stroke is Never Just Stroke
-
Feb 2, 2026, 16:48Seyed Mohsen Jahromi Moghadam: Antithrombotic Therapy After Transcatheter Structural Heart Interventions

