
The True Price of Gene Therapy: Breaking Down Beqvez’s $3.5M Cost and What Really Drives It
Scott Jeffers, Chief Technology Officer at GenSight Biologics, has shared a post on LinkedIn:
“The Real Cost of Gene Therapy Development: Beqvez as a Case Study
I’m often asked about the “outrageous” price of gene therapies. Let’s look at the real cost structure.
Beqvez (Pfizer’s hemophilia B gene therapy) launched at $3.5M. Here’s where that money actually goes:
𝗠𝗮𝗻𝘂𝗳𝗮𝗰𝘁𝘂𝗿𝗶𝗻𝗴 𝗥𝗲𝗮𝗹𝗶𝘁𝘆
Per batch cost (including QC): ~$2.0M
Drug Substance testing: $172K
Drug Product release: $123K
In-process testing: $135K
Stability testing: $227K
QC alone: $657K
Yes, nearly $700K per batch — and still not the biggest driver.
𝗧𝗵𝗲 𝗕𝗶𝗴𝗴𝗲𝗿 𝗣𝗶𝗰𝘁𝘂𝗿𝗲
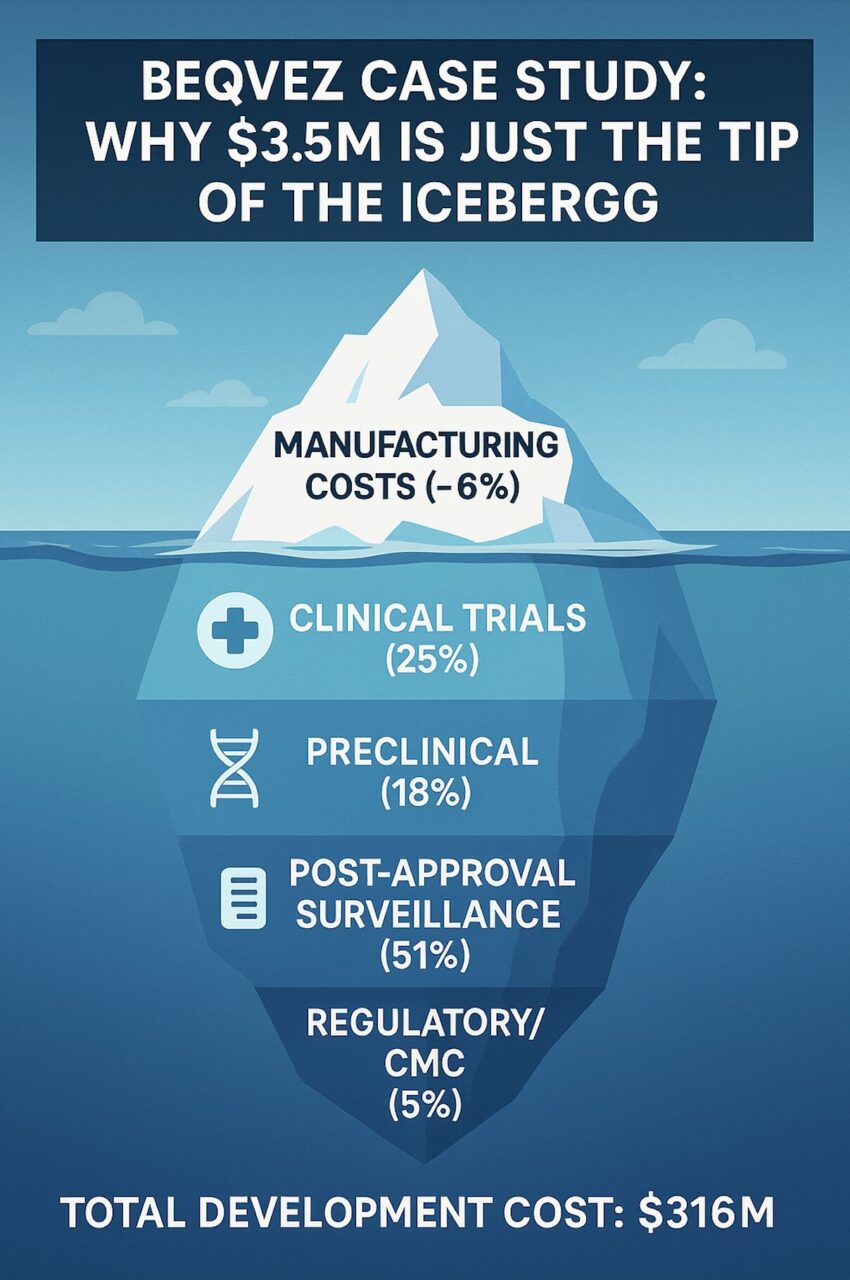
Total development cost: ~$316M
Clinical trials: $78M (25%)
Preclinical: $58M (18%)
Post-approval surveillance: $160M (51%)
CMC/Regulatory: $16M (5%)
Manufacturing per dose: $0.75M (<1%)
𝗧𝗵𝗲 𝗦𝘁𝘂𝗻𝗻𝗶𝗻𝗴 𝗧𝗿𝘂𝘁𝗵
Manufacturing + CMC = <6% of total costs.
The real spend is in:
Demonstrating safety & efficacy
15+ years of patient monitoring
Regulatory navigation
Building evidence for a novel therapy
𝗪𝗵𝘆 𝗜𝘁 𝗠𝗮𝘁𝘁𝗲𝗿𝘀
Pricing debates often blame manufacturing, but the economics come from:
Replacing $300K–$1M annual treatments with one-time interventions
Decades of risky R&D
Transformative patient outcomes
Note: Beqvez was discontinued in early 2025 — proof of how tough this market is, even when the science works.
𝗞𝗲𝘆 𝗧𝗮𝗸𝗲𝗮𝘄𝗮𝘆𝘀
Manufacturing optimization won’t fix pricing.
Clinical efficiency & regulatory innovation = biggest ROI.
Post-market surveillance costs are underestimated.
Platforms matter when manufacturing is <6%.
𝗪𝗵𝗮𝘁’𝘀 𝘆𝗼𝘂𝗿 𝗲𝘅𝗽𝗲𝗿𝗶𝗲𝗻𝗰𝗲? Are you seeing the same patterns?”
Stay updated with Hemostasis Today.
-
Feb 2, 2026, 17:44Important Webinar on Care for Patients with iTTP – ISTH
-
Feb 2, 2026, 17:21Tagreed Alkaltham: Some Risks Don’t Look Like Risks in Healthcare
-
Feb 2, 2026, 17:16Sifat Jubaira: Effect of Prolonged Tourniquet Application
-
Feb 2, 2026, 17:14Vivek Mahto: Understanding Deep Vein Thrombosis – Causes, Symptoms, and Prevention
-
Feb 2, 2026, 17:08Tareq Abadl: Heparin vs Warfarin
-
Feb 2, 2026, 17:07Mary Cushman: New Research on Aspirin Use in Pregnancy and Stroke Risk in Offspring
-
Feb 2, 2026, 16:52Aravind Palraj: Young Stroke is Never Just Stroke
-
Feb 2, 2026, 16:48Seyed Mohsen Jahromi Moghadam: Antithrombotic Therapy After Transcatheter Structural Heart Interventions
-
Feb 2, 2026, 16:45Shashank Joshi: Switching Among Oral Anticoagulants

