
ISTH 2025 Spotlight: HOPE-B Trial Highlights Sustained Efficacy of Gene Therapy for Hemophilia B
Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital, shared a post on X:
“ISTH 2025 Highlight: Our HOPE-B study results were brilliantly presented by Dr. Steven Pipe at ISTH 2025, showcasing 4-year durability data in haemophilia B gene therapy.
Study Overview
The phase 3 HOPE-B trial evaluated a single IV dose of etranacogene dezaparvovec (AAV5-FIX Padua) in adults with haemophilia B (FIX ≤2 IU/dL).
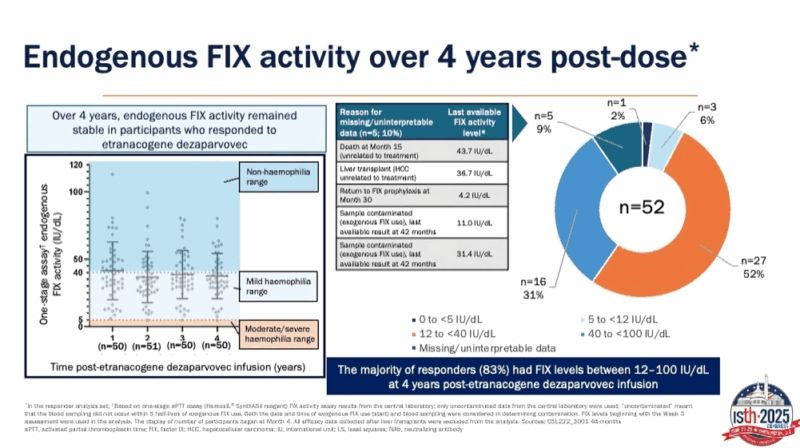
N = 54; Responders = 52
Key Efficacy Results
· ↓ 81% in annualized bleeding rate (ABR) (months 7–48 vs. baseline)
· ↓ 90% in ABR at Year 4 vs. lead-in period
· ↓ 98% in mean exogenous FIX use (IU/year) at Year 4
· 98% of participants remained prophylaxis-free through 4 years
· 37% required zero exogenous FIX infusions over 4 years
Factor IX (FIX) Expression
· Mean FIX activity: 37.4% at Year 4
· 83% of responders maintained FIX levels 12–100 IU/dL at 4 years
· Stable expression over 4 years → near-normal hemostasis
Safety Profile
· 96 treatment-related adverse events (TRAEs) over 4 years
· 96% occurred in first 6 months
· Most frequent: ALT↑ (18.5%), headache, flu-like illness
· No FIX inhibitors
· No thrombotic events
· No AAV5-associated genotoxicity or persistent late hepatotoxicity
Clinical Implications
· Single-dose gene therapy led to durable FIX expression and sustained reduction in bleeding and FIX use for ≥4 years
· Majority achieved and maintained prophylaxis freedom
· Favorable safety profile with minimal late events
· Ongoing follow-up remains important for long-term assessment
Congratulations to Steve Pipe and the co-authors.”
Stay updated with Hemostasis Today.
-
Jan 26, 2026, 04:59Abdul Mannan: BDUC – 4 Letters That Make Many Haematologists Uncomfortable
-
Jan 26, 2026, 04:51Manoj Kumar Singh: Power In Me Foundation Celebrates 2026 as Year Of Rare
-
Jan 26, 2026, 04:40Heghine Khachatryan: Did You Know VWD Comprises 3 Main Types?
-
Jan 25, 2026, 15:57Céline Chapelle Shares Clinical Predictors From the API-CAT Trial
-
Jan 25, 2026, 15:42Francesco Lo Monaco on Heart Disease Starting Quiet While Your Labs Speak First
-
Jan 25, 2026, 15:25Muhammad Ibrahim on Efficacy and Safety of Extended DOACs Use in VTE
-
Jan 25, 2026, 15:08Tushar Pandey on Managing Thrombotic Thrombocytopenic Purpura
-
Jan 25, 2026, 14:55Carolina Contreras Cuevas Shares a Nationwide Study on VTE in PAD
-
Jan 25, 2026, 14:40Jeannie Devereaux Links PRP and Physical Therapy
