
ADOPT Phase 4 Interim Analysis: Avatrombopag in Chronic ITP
Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital, posted on LinkedIn:
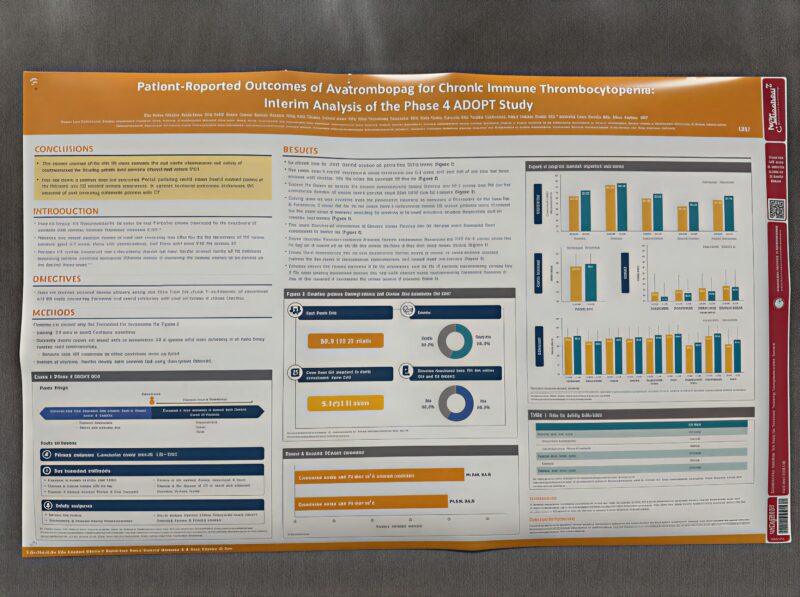
”ADOPT Phase 4 interim analysis: Avatrombopag in chronic ITP
Pleased to share this ASH poster as one of the co-authors. The interim dataset provides useful real-world evidence on avarombopag efficacy and safety in clinical practice.
Study overview:
– 199 patients | Mean 9.7 years from ITP diagnosis to enrollment
– 52.3 weeks median avarombopag dosing duration
– 44.1 cumulative weeks with platelet counts ≥30×10⁹/L
– 59.3% had received prior TPO-RA therapy—reflecting a population with established disease
Health-related quality of life outcomes:
→ EQ-5D-5L: improvements in mobility, usual activities, and pain/discomfort from enrollment to month 12
→ FACIT-Fatigue scores: consistent improvement across follow-up
→ WPAI: measurable reductions in work absenteeism, presenteeism, and overall impairment
→ ITP-PAQ: higher scores at month 12 across multiple scales
Safety findings:
18.1% adverse event rate | 12.6% serious AEs | 5.5% treatment-related AEs | 2.0% discontinued due to AEs
The data demonstrates sustained platelet control alongside functional improvements in work and daily activities. This aligns with our broader understanding of how effective haemostatic management translates to patient-reported outcomes.
The full analysis continues. Worth reviewing if you’re managing patients on TPO-RA therapy or evaluating treatment efficacy beyond platelet counts alone. ”
Stay updated with Hemostasis Today.
-
Jan 30, 2026, 11:38Aaron Rodriguez Calienes on Intracranial Stenting: Rescue vs First-Line Outcomes
-
Jan 30, 2026, 11:25Ahmed Nasreldein on Sex Disparities in Thrombolysis Delay Among Egyptian Stroke Patients
-
Jan 30, 2026, 11:17Yanki Yarman: My PhD Project Has Been Published in Blood!
-
Jan 30, 2026, 11:08Alexandros Apostolou on Complications Associated with IDU and Thrombophilia
-
Jan 30, 2026, 10:57Peisong Ma on GRK5 Polymorphism Affecting Platelets
-
Jan 30, 2026, 10:47Ashok Yadav Explains Fetal Thrombotic Vasculopathy: FTV
-
Jan 30, 2026, 10:34Saif ur Rahman Compares K2 and K3 EDTA: Key Differences Explained
-
Jan 30, 2026, 10:22Manpreet Gill on Heparin Resistance: When 14 Years of Experience Finally Gets a Plot Twist
-
Jan 30, 2026, 10:14Mohamed Elsaid on The Role of Hyperlipidemia in Thrombogenesis

