
Danny Hsu: Another Excellent Post from Wolfgang Miesbach – Comparing Low and Standard Dose Rituximab in iTTP
Danny Hsu, President of THANZ, reposted from Wolfgang Miesbach on LinkedIn:
“As DJ Khaled would say, “Another one!”…another excellent post from Wolfgang Miesbach showcasing an important study from Dr Mari Thomas from University College London Hospitals NHS Foundation Trust comparing low dose and standard dose rituximab in immune TTP!!!!
(Thank you Wolfgang, you have proven to me I do not need to attend ASH if you are there!)”
Quoting Wolfgang Miesbach’s post:
“ASH25 Data: Rituximab Dose Optimization in iTTP. Exciting results from Mari Thomas and colleagues on rituximab dosing strategies in immune thrombotic thrombocytopenic purpura (iTTP)—the first randomized trial comparing low-dose versus standard-dose preemptive rituximab in this life-threatening condition.
The Clinical Problem
iTTP remains devastating, with 74% relapse rates at 7 years in patients with persistent severe ADAMTS13 deficiency. While preemptive rituximab has transformed outcomes, optimal dosing remained uncertain—until now.
Study Design
- Standard dose: 375 mg/m² weekly × 4 weeks
- Low dose: 200 mg weekly × 4 weeks
- Population: 68 adults with iTTP in remission, ADAMTS13 activity ≤15%
- Primary objective: Non-inferiority for time to retreatment
Efficacy Outcomes and median time to retreatment:
Low dose: 19.7 months
Standard dose: 20.1 months
HR 0.93 (95% CI: 0.56-1.53), p=0.799
ADAMTS13 normalization: 31 days (low dose) vs. 21 days (standard dose)—HR 0.73, p=0.187
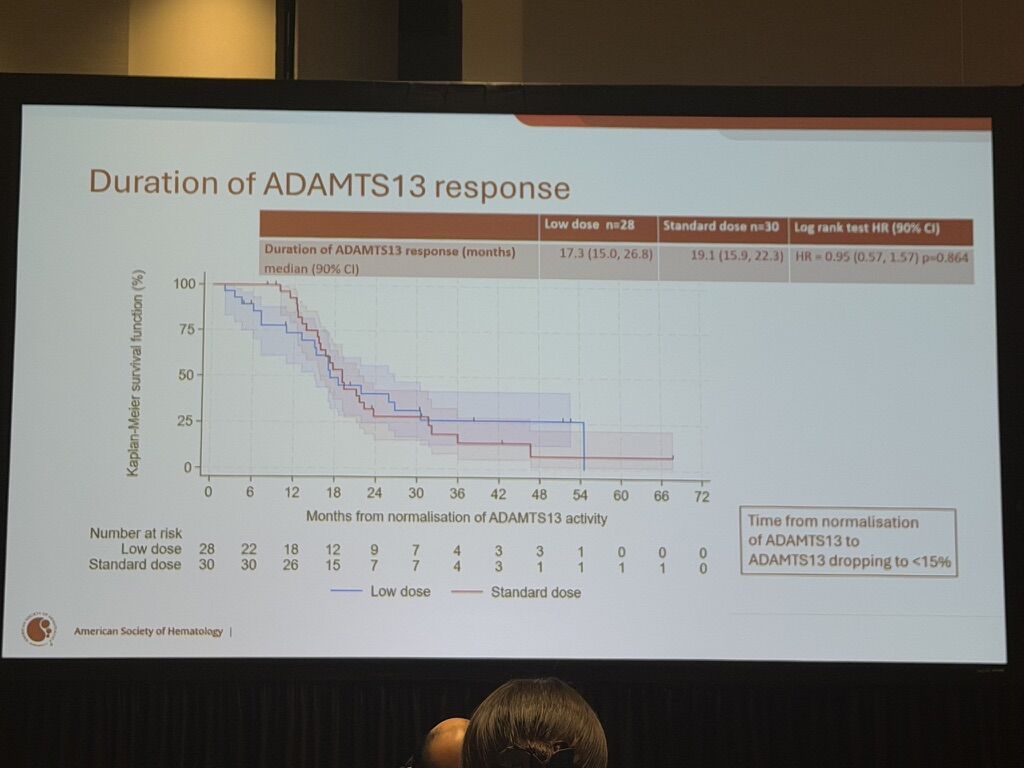
Duration of ADAMTS13 response: 17.3 months vs. 19.1 months (p=0.864)
Clinical relapse rates (remarkably low in both arms):
– Low dose: 6.3%
– Standard dose: 0%
Critical Treatment Effect Dynamics
Non-constant treatment effect: More retreatments observed in the first 12 months with low-dose rituximab
After 12 months: Curves converge—suggesting early vulnerability with dose reduction
Immune Reconstitution
B cell return: 12.2 months (low dose) vs. 16.1 months (standard dose), p=0.151
Safety Profile: Reassuring News
- Infusion reactions: Predominantly mild with both regimens
- Delayed adverse effects: Well-managed across both arms
- Pre-emptive rituximab remains safe, effective, and well-tolerated with repeated dosing
Results
This rigorous trial demonstrates that while low-dose rituximab shows activity, standard dosing (375 mg/m² weekly × 4) provides more consistent protection against early ADAMTS13 relapse—a critical insight for optimizing long-term management.
Recommendation: Standard-dose remains the preferred preemptive regimen in iTTP.”
Explore more posts from ASH2025 on Hemostasis Today.
-
Feb 23, 2026, 16:50Mutaz Al‑Sabah: Interesting Webinar on FH in Women is Now Available to Watch
-
Feb 23, 2026, 16:36Stéphanie Roullet։ New Method to Explore Primary Haemostasis in Cirrhotic Patients
-
Feb 23, 2026, 16:33Salvatore Massimo Petrina: Top 5 Game-Changers in the AHA and ACC PE Guidelines
-
Feb 23, 2026, 16:21Azin Alizadehasl: Introducing the Second Edition of a New Book on Echocardiography and Cardiac MRI
-
Feb 23, 2026, 16:16Kalyan Roy: When and Why Blood Irradiation Matters in Transfusion Medicine
-
Feb 23, 2026, 15:59Ney Carter Borges: Antithrombotic Therapy in 2025 – A Precision-Based Pharmacologic Update
-
Feb 23, 2026, 15:54Alan Nurden: From Early Discoveries to New Therapeutic Advances in von Willebrand Disease
-
Feb 23, 2026, 15:52Gevorg Tamamyan: Albania’s Vision for the Future of Pediatric Oncology
-
Feb 23, 2026, 15:36Simon Senanu: The Peripheral Blood Smear as an Essential Diagnostic Tool in Modern Medicine

