
Wolfgang Miesbach: New FVIII‑QQ Gene Therapy Aims for “Less is More” – Durable Expression with Enhanced Potency
Wolfgang Miesbach, Professor of Medicine at Frankfurt University Hospital, shared on LinkedIn:
“Less is more: How enhanced‑function FVIII‑QQ can optimize a low‑dose gene therapy approach for haemophilia A. Great ASH presentation by Nina Frey. SPK‑8011QQ leverages the established SPK‑8011 capsid (dirloctocogene samoparvovec), which in Phase 1/2 trials demonstrated:
- Up to 6.5 years of durable FVIII expression within the mild HA range
- No wide expression variability across time points
- No sustained or late ALT elevations
Shortened corticosteroid exposure with IVMP; return to prophylaxis in only 5 of 25 participants
Now combining this proven durability and safety profile with the enhanced functional potency of FVIII‑QQ.
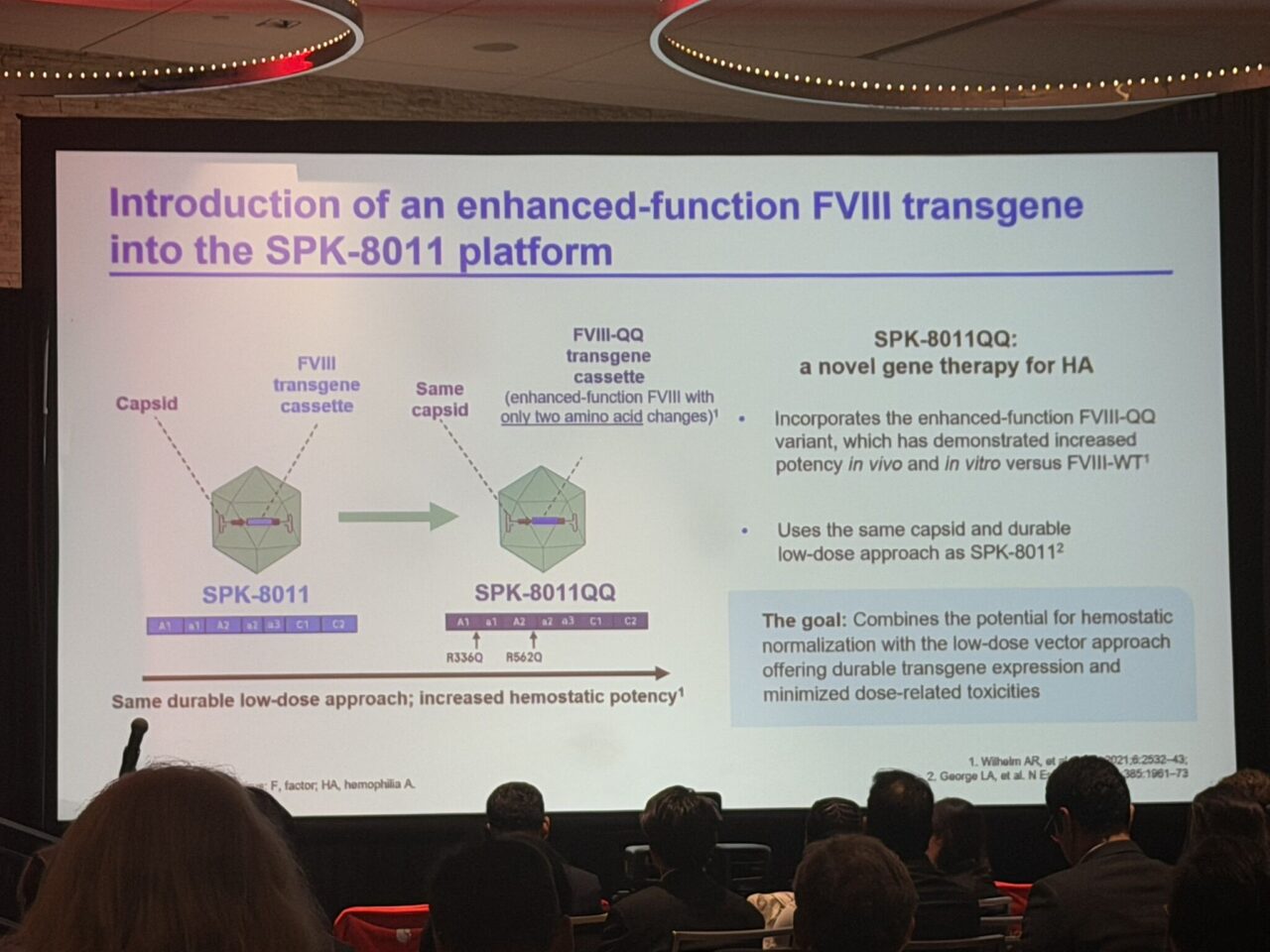
Introducing SPK‑8011QQ: This novel construct incorporates FVIII‑QQ – an enhanced‑function FVIII variant with just two amino‑acid substitutions (R336Q and R562Q) that:
- Confer resistance to activated protein C (aPC) cleavage
- Preserve physiological A2‑domain dissociation (maintaining natural control mechanisms).
Enhanced potency in the presence of aPC:
– FVIII‑QQ retained ~65% residual activity in chromogenic assays (vs. ~43% for FVIII‑WT) at 10 nM aPC
– Significantly higher residual peak thrombin across aPC concentrations in thrombin generation assays (p=0.0018 at 4 nM)
– 80–100% FVIII activity equivalent in mouse ex vivo plasma, with markedly enhanced thrombin generation vs. FVIII‑WT
Superior haemostatic control at lower FVIII activity levels:
Tail‑clip bleed model: FVIII‑QQ achieved enhanced bleed control at markedly lower FVIII levels than FVIII‑WT, with significantly reduced blood loss (p=0.0089 and p=0.0079)
Encouraging preliminary safety profile:
Ferric chloride thrombosis model: FVIII‑QQ showed a lower incidence of animals reaching the near‑occlusive zone (>85% blood flow reduction) vs. positive control
A Phase 2b study by Roche is now underway to translate these preclinical insights into meaningful clinical benefit.”
Read more from Wolfgang Miesbach on Hemostasis Today.
-
Jan 31, 2026, 16:35IV Thrombolysis Does Not Improve Vision in Acute CRAO: Ahmed Koriesh on TenCRAOS Study
-
Jan 31, 2026, 16:24Joyce John Chalakkal: The Dangerous Area of The Face….
-
Jan 31, 2026, 16:04Rayya Saadiq Reflects On Qatar Health Congress 2026
-
Jan 31, 2026, 15:12Amar Raval on Oral Thin Film Drug Delivery Systems for Thrombosis Therapy
-
Jan 31, 2026, 14:40Heghine Khachatryan on WFH’s Call to Advance Health Equity for People with Bleeding Disorders
-
Jan 31, 2026, 14:10Emilia Arturo: Snake Venom, Clotting, and CryoEM!
-
Jan 31, 2026, 08:13Ming Y Lim On The Value of National Mentorship
-
Jan 31, 2026, 06:37Christian Schulze Presents The Post-Hoc Analysis of the DanGer Shock Trial
-
Jan 31, 2026, 06:27Negative Trials Matter: Anne Hege Aamodt Shares The TenCRAOS Trial

